Expression of Microcystis Biosynthetic Gene Clusters in Natural Populations Suggests Temporally Dynamic Synthesis of Novel and Known Secondary Metabolites in Western Lake Erie
- PMID: 37070981
- PMCID: PMC10231183
- DOI: 10.1128/aem.02092-22
Expression of Microcystis Biosynthetic Gene Clusters in Natural Populations Suggests Temporally Dynamic Synthesis of Novel and Known Secondary Metabolites in Western Lake Erie
Abstract
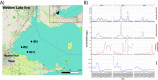
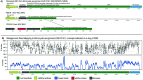
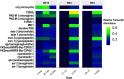
Microcystis spp. produce diverse secondary metabolites within freshwater cyanobacterial harmful algal blooms (cyanoHABs) around the world. In addition to the biosynthetic gene clusters (BGCs) encoding known compounds, Microcystis genomes harbor numerous BGCs of unknown function, indicating a poorly understood chemical repertoire. While recent studies show that Microcystis produces several metabolites in the lab and field, little work has focused on analyzing the abundance and expression of its broader suite of BGCs during cyanoHAB events. Here, we use metagenomic and metatranscriptomic approaches to track the relative abundance of Microcystis BGCs and their transcripts throughout the 2014 western Lake Erie cyanoHAB. The results indicate the presence of several transcriptionally active BGCs that are predicted to synthesize both known and novel secondary metabolites. The abundance and expression of these BGCs shifted throughout the bloom, with transcript abundance levels correlating with temperature, nitrate, and phosphorus concentrations and the abundance of co-occurring predatory and competitive eukaryotic microorganisms, suggesting the importance of both abiotic and biotic controls in regulating expression. This work highlights the need for understanding the chemical ecology and potential risks to human and environmental health posed by secondary metabolites that are produced but often unmonitored. It also indicates the prospects for identifying pharmaceutical-like molecules from cyanoHAB-derived BGCs. IMPORTANCE Microcystis spp. dominate cyanobacterial harmful algal blooms (cyanoHABs) worldwide and pose significant threats to water quality through the production of secondary metabolites, many of which are toxic. While the toxicity and biochemistry of microcystins and several other compounds have been studied, the broader suite of secondary metabolites produced by Microcystis remains poorly understood, leaving gaps in our understanding of their impacts on human and ecosystem health. We used community DNA and RNA sequences to track the diversity of genes encoding synthesis of secondary metabolites in natural Microcystis populations and assess patterns of transcription in western Lake Erie cyanoHABs. Our results reveal the presence of both known gene clusters that encode toxic secondary metabolites as well as novel ones that may encode cryptic compounds. This research highlights the need for targeted studies of the secondary metabolite diversity in western Lake Erie, a vital freshwater source to the United States and Canada.
Keywords: Lake Erie; Microcystis; cyanoHABs; metagenomics; metatranscriptomics; secondary metabolites; strain diversity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Steffen MM, Davis TW, McKay RML, Bullerjahn GS, Krausfeldt LE, Stough JMA, Neitzey ML, Gilbert NE, Boyer GL, Johengen TH, Gossiaux DC, Burtner AM, Palladino D, Rowe MD, Dick GJ, Meyer KA, Levy S, Boone BE, Stumpf RP, Wynne TT, Zimba PV, Gutierrez D, Wilhelm SW. 2017. Ecophysiological examination of the Lake Erie microcystis bloom in 2014: linkages between biology and the water supply shutdown of Toledo, OH. Environ Sci Technol 51:6745–6755. doi: 10.1021/acs.est.7b00856. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

