Longitudinal Follow-Up of the Immunity to SARS-CoV-2 in Health Care Workers in Argentina: Persistence of Humoral Response and Neutralizing Capacity after Sputnik V Vaccination
- PMID: 37070983
- PMCID: PMC10286708
- DOI: 10.1128/msphere.00662-22
Longitudinal Follow-Up of the Immunity to SARS-CoV-2 in Health Care Workers in Argentina: Persistence of Humoral Response and Neutralizing Capacity after Sputnik V Vaccination
Abstract
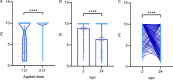
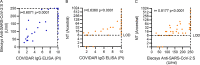
SARS-CoV-2 vaccine protection has encountered waning of immune response and breakthrough infections. The hybrid immune response generated by the combination of vaccination and infection was shown to offer higher and broader protection. Here, we present a seroprevalence study of anti-SARS-CoV-2 spike/RBD IgG in 1,121 health care workers immunized with Sputnik V and a follow-up of humoral response at 2 and 24 weeks postvaccination (wpv), including neutralizing antibody response (NAT) against ancestral, Gamma, and Delta variants. The first seroprevalence study showed that among 122 individuals with one dose, 90.2% were seropositive versus 99.7% seropositivity among volunteers with the complete two-dose regimen. At 24 wpv, 98.7% of the volunteers remained seropositive, although antibody levels decreased. IgG levels and NAT were higher in individuals that had acquired COVID-19 previous to vaccination than in naive individuals at 2 and 24 wpv. Antibody levels dropped over time in both groups. In contrast, IgG levels and NAT increased after vaccine breakthrough infection. At 2 wpv, 35/40 naive individuals had detectable NAT against SARS-CoV-2 Gamma and 6/40 against Delta. In turn, 8/9 previously infected individuals developed a neutralizing response against SARS-CoV-2 Gamma and 4/9 against Delta variants. NAT against variants followed a trajectory similar to NAT against ancestral SARS-CoV-2, and breakthrough infection led to an increase in NAT and complete seroconversion against variants. In conclusion, Sputnik V-induced humoral response persisted at 6 months postvaccination, and hybrid immunity induced higher levels of anti-S/RBD antibodies and NAT in previously exposed individuals, boosted the response after vaccination, and conferred wider breadth of protection. IMPORTANCE Since December 2020, Argentina has begun a mass vaccination program. The first vaccine available in our country was Sputnik V, which has been approved for use in 71 countries with a total population of 4 billion people. Despite all the available information, there are fewer published studies on the response induced by Sputnik V vaccination compared to that of other vaccines. Although the global political context has paralyzed the verification by the WHO of the efficacy of this vaccine, our work aims to add new clear and necessary evidence to Sputnik V performance. Our results contribute to general knowledge of the humoral immune response developed by vaccines based on viral vector technology, highlighting the higher immune protection conferred by hybrid immunity and reinforcing the importance of completing vaccination schedules and booster doses to maintain adequate antibody levels.
Keywords: SARS-CoV-2; Sputnik V; humoral immune response; hybrid immunity; vaccination.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Longitudinal Study after Sputnik V Vaccination Shows Durable SARS-CoV-2 Neutralizing Antibodies and Reduced Viral Variant Escape to Neutralization over Time.mBio. 2022 Feb 22;13(1):e0344221. doi: 10.1128/mbio.03442-21. Epub 2022 Jan 25. mBio. 2022. PMID: 35073758 Free PMC article.
-
Humoral and cellular response of two different vaccines against SARS-CoV-2 in a group of healthcare workers: An observational study.J Immunol Methods. 2024 May;528:113665. doi: 10.1016/j.jim.2024.113665. Epub 2024 Mar 14. J Immunol Methods. 2024. PMID: 38490578
-
Sputnik V vaccine elicits seroconversion and neutralizing capacity to SARS-CoV-2 after a single dose.Cell Rep Med. 2021 Aug 17;2(8):100359. doi: 10.1016/j.xcrm.2021.100359. Epub 2021 Jul 9. Cell Rep Med. 2021. PMID: 34308389 Free PMC article.
-
Infection-mediated immune response in SARS-CoV-2 breakthrough infection and implications for next-generation COVID-19 vaccine development.Vaccine. 2024 Feb 27;42(6):1401-1406. doi: 10.1016/j.vaccine.2024.01.088. Epub 2024 Feb 2. Vaccine. 2024. PMID: 38310015 Review.
-
Unravelling humoral immunity in SARS-CoV-2: Insights from infection and vaccination.Hum Antibodies. 2024;32(3):85-106. doi: 10.3233/HAB-230017. Hum Antibodies. 2024. PMID: 38758995 Review.
References
-
- Logunov DY, Dolzhikova IV, Zubkova OV, Tukhvatullin AI, Shcheblyakov DV, Dzharullaeva AS, Grousova DM, Erokhova AS, Kovyrshina AV, Botikov AG, Izhaeva FM, Popova O, Ozharovskaya TA, Esmagambetov IB, Favorskaya IA, Zrelkin DI, Voronina DV, Shcherbinin DN, Semikhin AS, Simakova YV, Tokarskaya EA, Lubenets NL, Egorova DA, Shmarov MM, Nikitenko NA, Morozova LF, Smolyarchuk EA, Kryukov EV, Babira VF, Borisevich SV, Naroditsky BS, Gintsburg AL. 2020. Safety and immunogenicity of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine in two formulations: two open, non-randomised phase 1/2 studies from Russia. Lancet 396:887–897. doi:10.1016/S0140-6736(20)31866-3. - DOI - PMC - PubMed
-
- Logunov DY, Dolzhikova IV, Shcheblyakov DV, Tukhvatulin AI, Zubkova OV, Dzharullaeva AS, Kovyrshina AV, Lubenets NL, Grousova DM, Erokhova AS, Botikov AG, Izhaeva FM, Popova O, Ozharovskaya TA, Esmagambetov IB, Favorskaya IA, Zrelkin DI, Voronina DV, Shcherbinin DN, Semikhin AS, Simakova YV, Tokarskaya EA, Egorova DA, Shmarov MM, Nikitenko NA, Gushchin VA, Smolyarchuk EA, Zyryanov SK, Borisevich SV, Naroditsky BS, Gintsburg AL, Gam-COVID-Vac Vaccine Trial Group . 2021. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: an interim analysis of a randomised controlled phase 3 trial in Russia. Lancet 397:671–681. doi:10.1016/S0140-6736(21)00234-8. - DOI - PMC - PubMed
-
- Russian Direct Investment Fund. 2003. About Sputnik V. https://sputnikvaccine.com/about-vaccine/.
-
- Widge AT, Rouphael NG, Jackson LA, Anderson EJ, Roberts PC, Makhene M, Chappell JD, Denison MR, Stevens LJ, Pruijssers AJ, McDermott AB, Flach B, Lin BC, Doria-Rose NA, O'Dell S, Schmidt SD, Neuzil KM, Bennett H, Leav B, Makowski M, Albert J, Cross K, Edara V-V, Floyd K, Suthar MS, Buchanan W, Luke CJ, Ledgerwood JE, Mascola JR, Graham BS, Beigel JH, mRNA-1273 Study Group . 2021. Durability of responses after SARS-CoV-2 mRNA-1273 vaccination. N Engl J Med 384:80–82. doi:10.1056/NEJMc2032195. - DOI - PMC - PubMed
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

