Middle Meningeal Artery Embolization for Chronic Subdural Hematoma: Predictors of Clinical and Radiographic Failure from 636 Embolizations
- PMID: 37070990
- PMCID: PMC10323293
- DOI: 10.1148/radiol.222045
Middle Meningeal Artery Embolization for Chronic Subdural Hematoma: Predictors of Clinical and Radiographic Failure from 636 Embolizations
Abstract
Background Knowledge regarding predictors of clinical and radiographic failures of middle meningeal artery (MMA) embolization (MMAE) treatment for chronic subdural hematoma (CSDH) is limited. Purpose To identify predictors of MMAE treatment failure for CSDH. Materials and Methods In this retrospective study, consecutive patients who underwent MMAE for CSDH from February 2018 to April 2022 at 13 U.S. centers were included. Clinical failure was defined as hematoma reaccumulation and/or neurologic deterioration requiring rescue surgery. Radiographic failure was defined as a maximal hematoma thickness reduction less than 50% at last imaging (minimum 2 weeks of head CT follow-up). Multivariable logistic regression models were constructed to identify independent failure predictors, controlling for age, sex, concurrent surgical evacuation, midline shift, hematoma thickness, and pretreatment baseline antiplatelet and anticoagulation therapy. Results Overall, 530 patients (mean age, 71.9 years ± 12.8 [SD]; 386 men; 106 with bilateral lesions) underwent 636 MMAE procedures. At presentation, the median CSDH thickness was 15 mm and 31.3% (166 of 530) and 21.7% (115 of 530) of patients were receiving antiplatelet and anticoagulation medications, respectively. Clinical failure occurred in 36 of 530 patients (6.8%, over a median follow-up of 4.1 months) and radiographic failure occurred in 26.3% (137 of 522) of procedures. At multivariable analysis, independent predictors of clinical failure were pretreatment anticoagulation therapy (odds ratio [OR], 3.23; P = .007) and an MMA diameter less than 1.5 mm (OR, 2.52; P = .027), while liquid embolic agents were associated with nonfailure (OR, 0.32; P = .011). For radiographic failure, female sex (OR, 0.36; P = .001), concurrent surgical evacuation (OR, 0.43; P = .009), and a longer imaging follow-up time were associated with nonfailure. Conversely, MMA diameter less than 1.5 mm (OR, 1.7; P = .044), midline shift (OR, 1.1; P = .02), and superselective MMA catheterization (without targeting the main MMA trunk) (OR, 2; P = .029) were associated with radiographic failure. Sensitivity analyses retained these associations. Conclusion Multiple independent predictors of failure of MMAE treatment for chronic subdural hematomas were identified, with small diameter (<1.5 mm) being the only factor independently associated with both clinical and radiographic failures. © RSNA, 2023 Supplemental material is available for this article. See also the editorial by Chaudhary and Gemmete in this issue.
Conflict of interest statement
Figures

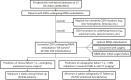




Comment in
-
Insights into Middle Meningeal Artery Embolization in Chronic Subdural Hematoma: What Does Not Work.Radiology. 2023 May;307(4):e230405. doi: 10.1148/radiol.230405. Epub 2023 Apr 18. Radiology. 2023. PMID: 37070993 No abstract available.
References
-
- Ducruet AF , Grobelny BT , Zacharia BE , et al. . The surgical management of chronic subdural hematoma . Neurosurg Rev 2012. ; 35 ( 2 ): 155 – 169 ; discussion 169 . - PubMed
-
- Miranda LB , Braxton E , Hobbs J , Quigley MR . Chronic subdural hematoma in the elderly: not a benign disease . J Neurosurg 2011. ; 114 ( 1 ): 72 – 76 . - PubMed
-
- Ivamoto HS , Lemos HP Jr , Atallah AN . Surgical treatments for chronic subdural hematomas: a comprehensive systematic review . World Neurosurg 2016. ; 86 : 399 – 418 . - PubMed
-
- Liu W , Bakker NA , Groen RJ . Chronic subdural hematoma: a systematic review and meta-analysis of surgical procedures . J Neurosurg 2014. ; 121 ( 3 ): 665 – 673 . - PubMed
-
- Ironside N , Nguyen C , Do Q , et al. . Middle meningeal artery embolization for chronic subdural hematoma: a systematic review and meta-analysis . J Neurointerv Surg 2021. ; 13 ( 10 ): 951 – 957 . - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

