Independent Effects of HIV and Antiretroviral Therapy on the Oral Microbiome Identified by Multivariate Analyses
- PMID: 37071004
- PMCID: PMC10294613
- DOI: 10.1128/mbio.00409-23
Independent Effects of HIV and Antiretroviral Therapy on the Oral Microbiome Identified by Multivariate Analyses
Abstract
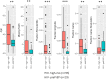
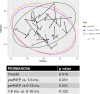
The oral microbiome is an important predictor of health and disease. We recently reported significant yet modest effects of HIV under highly active antiretroviral therapy (ART) on the oral microbiome (bacterial and fungal) in a large cohort of HIV-positive (HIV+) and matched HIV-negative (HIV-) individuals. As it was unclear whether ART added to or masked further effects of HIV on the oral microbiome, the present study aimed to analyze the effects of HIV and ART independently, which also included HIV- subjects on preexposure prophylaxis (PrEP) therapy. Cross-sectional analyses of the effect of HIV devoid of ART (HIV+ ART- versus matched HIV- subjects) showed a significant effect on both the bacteriome and mycobiome (P < 0.024) after controlling for other clinical variables (permutational multivariate analysis of variance [PERMANOVA] of Bray-Curtis dissimilarity). Cross-sectional analyses evaluating the effects of ART (HIV+ ART+ versus HIV+ ART- subjects) revealed a significant effect on the mycobiome (P < 0.007) but not the bacteriome. In parallel longitudinal analyses, ART (before versus after the initiation of ART) had a significant effect on the bacteriome, but not the mycobiome, of HIV+ and HIV- PrEP subjects (P < 0.005 and P < 0.016, respectively). These analyses also revealed significant differences in the oral microbiome and several clinical variables between HIV- PrEP subjects (pre-PrEP) and the HIV-matched HIV- group (P < 0.001). At the species level, a small number of differences in both bacterial and fungal taxa were identified within the effects of HIV and/or ART. We conclude that the effects of HIV and ART on the oral microbiome are similar to those of the clinical variables but collectively are modest overall. IMPORTANCE The oral microbiome can be an important predictor of health and disease. For persons living with HIV (PLWH), HIV and highly active antiretroviral therapy (ART) may have a significant influence on their oral microbiome. We previously reported a significant effect of HIV with ART on both the bacteriome and mycobiome. It was unclear whether ART added to or masked further effects of HIV on the oral microbiome. Hence, it was important to evaluate the effects of HIV and ART independently. For this, multivariate cross-sectional and longitudinal oral microbiome analyses (bacteriome and mycobiome) were conducted within the cohort, including HIV+ ART+ subjects and HIV+ and HIV- (preexposure prophylaxis [PrEP]) subjects before and after the initiation of ART. While we report independent significant effects of HIV and ART on the oral microbiome, we conclude that their influence is similar to that of the clinical variables but collectively modest overall.
Keywords: ART; HIV; bacteriome; mycobiome; oral microbiome.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Effect of HIV/HAART and Other Clinical Variables on the Oral Mycobiome Using Multivariate Analyses.mBio. 2021 Mar 23;12(2):e00294-21. doi: 10.1128/mBio.00294-21. mBio. 2021. PMID: 33758093 Free PMC article.
-
The palatine tonsil bacteriome, but not the mycobiome, is altered in HIV infection.BMC Microbiol. 2018 Oct 5;18(1):127. doi: 10.1186/s12866-018-1274-9. BMC Microbiol. 2018. PMID: 30290791 Free PMC article.
-
Oral and Gut Microbial Diversity and Immune Regulation in Patients with HIV on Antiretroviral Therapy.mSphere. 2020 Feb 5;5(1):e00798-19. doi: 10.1128/mSphere.00798-19. mSphere. 2020. PMID: 32024712 Free PMC article.
-
Neglected mycobiome in HIV infection: Alterations, common fungal diseases and antifungal immunity.Front Immunol. 2022 Nov 10;13:1015775. doi: 10.3389/fimmu.2022.1015775. eCollection 2022. Front Immunol. 2022. PMID: 36439143 Free PMC article. Review.
-
The mycobiome in HIV.Curr Opin HIV AIDS. 2018 Jan;13(1):69-72. doi: 10.1097/COH.0000000000000432. Curr Opin HIV AIDS. 2018. PMID: 29028668 Free PMC article. Review.
Cited by
-
Impacts of Antiretroviral Therapy on the Oral Microbiome and Periodontal Health of Feline Immunodeficiency Virus-Positive Cats.Viruses. 2025 Feb 13;17(2):257. doi: 10.3390/v17020257. Viruses. 2025. PMID: 40007012 Free PMC article.
-
Fungi and bacteria occupy distinct spatial niches within carious dentin.PLoS Pathog. 2024 May 28;20(5):e1011865. doi: 10.1371/journal.ppat.1011865. eCollection 2024 May. PLoS Pathog. 2024. PMID: 38805482 Free PMC article.
-
Oral Microbiome and Dental Caries in Kenyan Children and Adolescents Living with HIV.JDR Clin Trans Res. 2025 Feb 6;10(4):23800844241311862. doi: 10.1177/23800844241311862. Online ahead of print. JDR Clin Trans Res. 2025. PMID: 39915906 Free PMC article.
-
Impacts of Antiretroviral Therapy on the Oral Microbiome and Periodontal Health of Feline Immunodeficiency Virus Positive Cats.bioRxiv [Preprint]. 2024 Jul 11:2024.07.10.602918. doi: 10.1101/2024.07.10.602918. bioRxiv. 2024. Update in: Viruses. 2025 Feb 13;17(2):257. doi: 10.3390/v17020257. PMID: 39026780 Free PMC article. Updated. Preprint.
-
Human immunodeficiency virus and oral microbiota: mutual influence on the establishment of a viral gingival reservoir in individuals under antiretroviral therapy.Front Cell Infect Microbiol. 2024 Apr 10;14:1364002. doi: 10.3389/fcimb.2024.1364002. eCollection 2024. Front Cell Infect Microbiol. 2024. PMID: 38660490 Free PMC article. Review.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
