Peripheral Nerve Injury Induced by Japanese Encephalitis Virus in C57BL/6 Mouse
- PMID: 37071015
- PMCID: PMC10231255
- DOI: 10.1128/jvi.01658-22
Peripheral Nerve Injury Induced by Japanese Encephalitis Virus in C57BL/6 Mouse
Abstract
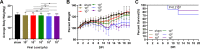
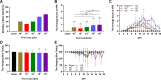
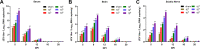
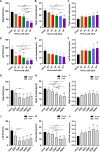
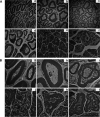
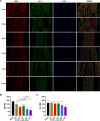
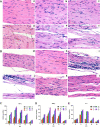
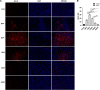
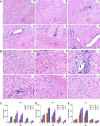
Japanese encephalitis virus (JEV), with neurotoxic and neuroinvasive properties, is the major cause of human viral encephalitis in Asia. Although Guillain-Barré syndrome caused by JEV infections is not frequent, a few cases have been reported in recent years. To date, no existing animal model for JEV-induced peripheral nerve injury (PNI) has been established, and thus the pathogenic mechanism is not clarified. Therefore, an animal model is urgently required to clarify the correlation between JEV infection and PNI. In the present study, we used JEV GIb strain of NX1889 to establish a mouse model of JEV infection. The general neurological signs emerged on day 3 of modeling. The motor function continued to deteriorate, reaching a maximum at 8 to 13 days postinfection (dpi) and gradually recovered after 16 dpi. The injuries of 105 PFU and 106 PFU groups were the most severe. Transmission electron microscopy and immunofluorescence staining showed varying degrees of demyelination and axonal degeneration in the sciatic nerves. The electrophysiological recordings demonstrated the presence of demyelinating peripheral neuropathy with reduced nerve conduction velocity. The decreased amplitudes and the prolonged end latency revealed axonal-type motor neuropathy. Demyelination is predominant in the early stage, followed by axonal injury. The expression level of JEV-E protein and viral RNA was elevated in the injured sciatic nerves, suggesting that it may cause PNI at the early stage. Inflammatory cell infiltration and increased inflammatory cytokines indicated that neuroinflammation is involved in JEV-induced PNI. IMPORTANCE JEV is a neurotropic flavivirus belonging to the Flaviviridae family and causes high mortality and disability rates. It invades the central nervous system and induces acute inflammatory injury and neuronal death. Thus, JEV infection is a major global public health concern. Previously, motor dysfunction was mainly attributed to central nervous system damage. Our knowledge regarding JEV-induced PNI is vague and neglected. Therefore, a laboratory animal model is essential. Herein, we showed that C57BL/6 mice can be used to study JEV-induced PNI through multiple approaches. We also demonstrated that viral loads might be positively correlated with lesion severity. Therefore, inflammation and direct virus infection may be the putative mechanisms underlying JEV-induced PNI. The results of this study laid the foundation for further elucidation of the pathogenesis mechanisms of PNI caused by JEV.
Keywords: C57BL/6 mouse; JEV; PNI; axon; myelin sheath; sciatic nerves.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Li F, Wang Y, Yu L, Cao S, Wang K, Yuan J, Wang C, Wang K, Cui M, Fu ZF. 2015. Viral infection of the central nervous system and neuroinflammation precede blood-brain barrier disruption during Japanese encephalitis virus infection. J Virol 89:5602–5614. doi:10.1128/JVI.00143-15. - DOI - PMC - PubMed
-
- Liu W, Fu S, Ma X, Chen X, Wu D, Zhou L, Yin Q, Li F, He Y, Lei W, Li Y, Xu S, Wang H, Wang Z, Wang H, Yu H, Liang G. 2020. An outbreak of Japanese encephalitis caused by genotype Ib Japanese encephalitis virus in China, 2018: a laboratory and field investigation. PLoS Negl Trop Dis 14:e0008312. doi:10.1371/journal.pntd.0008312. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

