Quantitative 1H Nuclear Magnetic Resonance Assay for the Rapid Detection of Pyrazinamide Resistance in Mycobacterium tuberculosis from Sputum Samples
- PMID: 37071032
- PMCID: PMC10204627
- DOI: 10.1128/jcm.01522-22
Quantitative 1H Nuclear Magnetic Resonance Assay for the Rapid Detection of Pyrazinamide Resistance in Mycobacterium tuberculosis from Sputum Samples
Abstract
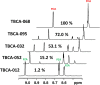
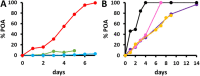
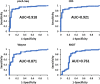
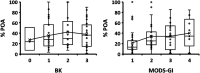
Tuberculosis (TB), caused by Mycobacterium tuberculosis, is one of the 10 leading killer diseases in the world. At least one-quarter of the population has been infected, and there are 1.3 million deaths annually. The emergence of multidrug-resistant (MDR) and extensively drug-resistant (XDR) strains challenges TB treatments. One of the drugs widely used in first- and second-line regimens is pyrazinamide (PZA). Statistically, 50% of MDR and 90% of XDR clinical strains are resistant to PZA, and recent studies have shown that its use in patients with PZA-resistant strains is associated with higher mortality rates. Therefore, the is an urgent need for the development of an accurate and efficient PZA susceptibility assay. PZA crosses the M. tuberculosis membrane and is hydrolyzed to its active form, pyrazinoic acid (POA), by a nicotinamidase encoded by the pncA gene. Up to 99% of clinical PZA-resistant strains have mutations in this gene, suggesting that this is the most likely mechanism of resistance. However, not all pncA mutations confer PZA resistance, only the ones that lead to limited POA production. Therefore, susceptibility to PZA may be addressed simply by its ability to form, or not, POA. Here, we present a nuclear magnetic resonance method to accurately quantify POA directly in the supernatant of sputum cultures collected from TB patients. The ability of the clinical sputum culture to hydrolyze PZA was determined, and the results were correlated with the results of other biochemical and molecular PZA drug susceptibility assays. The excellent sensitivity and specificity values attained suggest that this method could become the new gold standard for the determination of PZA susceptibility.
Keywords: Mycobacterium tuberculosis; antibiotic resistance; clinical trial; drug susceptibility test; nuclear magnetic resonance; pyrazinamide; tuberculosis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- World Health Organization. 2021. Global tuberculosis report. World Health Organization, Geneva, Switzerland.
-
- Hu Y, Coates AR, Mitchison DA. 2006. Sterilising action of pyrazinamide in models of dormant and rifampicin-tolerant Mycobacterium tuberculosis. Int J Tuberc Lung Dis 10:317–322. - PubMed
-
- Malome L, Schurr A, Lindh H, McKenzie D, Kiser JS, Williams JH. 1952. The effect of pyrazinamide (aldinamide) on experimental tuberculosis in mice. Am Rev Tuberc 65:511–518. - PubMed
-
- Yeager RL, Munroe WG, Dessau FI. 1952. Pyrazinamide (aldinamide*) in the treatment of pulmonary tuberculosis. Trans Annu Meet Natl Tuberc Assoc 48:178–201. - PubMed
-
- Fox W, Ellard GA, Mitchison DA. 1999. Studies on the treatment of tuberculosis undertaken by the British Medical Research Council tuberculosis units, 1946-1986, with relevant subsequent publications. Int J Tuberc Lung Dis 3:S231–S279. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

