Panitumumab vs Bevacizumab Added to Standard First-line Chemotherapy and Overall Survival Among Patients With RAS Wild-type, Left-Sided Metastatic Colorectal Cancer: A Randomized Clinical Trial
- PMID: 37071094
- PMCID: PMC10114040
- DOI: 10.1001/jama.2023.4428
Panitumumab vs Bevacizumab Added to Standard First-line Chemotherapy and Overall Survival Among Patients With RAS Wild-type, Left-Sided Metastatic Colorectal Cancer: A Randomized Clinical Trial
Erratum in
-
Error in Text Wording and Incorrect Reference.JAMA. 2023 Jun 27;329(24):2196. doi: 10.1001/jama.2023.10533. JAMA. 2023. PMID: 37367990 Free PMC article. No abstract available.
Abstract
Importance: For patients with RAS wild-type metastatic colorectal cancer, adding anti-epidermal growth factor receptor (anti-EGFR) or anti-vascular endothelial growth factor (anti-VEGF) monoclonal antibodies to first-line doublet chemotherapy is routine, but the optimal targeted therapy has not been defined.
Objective: To evaluate the effect of adding panitumumab (an anti-EGFR monoclonal antibody) vs bevacizumab (an anti-VEGF monoclonal antibody) to standard first-line chemotherapy for treatment of RAS wild-type, left-sided, metastatic colorectal cancer.
Design, setting, and participants: Randomized, open-label, phase 3 clinical trial at 197 sites in Japan in May 2015-January 2022 among 823 patients with chemotherapy-naive RAS wild-type, unresectable metastatic colorectal cancer (final follow-up, January 14, 2022).
Interventions: Panitumumab (n = 411) or bevacizumab (n = 412) plus modified fluorouracil, l-leucovorin, and oxaliplatin (mFOLFOX6) every 14 days.
Main outcomes and measures: The primary end point, overall survival, was tested first in participants with left-sided tumors, then in the overall population. Secondary end points were progression-free survival, response rate, duration of response, and curative (defined as R0 status) resection rate.
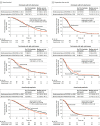
Results: In the as-treated population (n = 802; median age, 66 years; 282 [35.2%] women), 604 (75.3%) had left-sided tumors. Median follow-up was 61 months. Median overall survival was 37.9 months with panitumumab vs 34.3 months with bevacizumab in participants with left-sided tumors (hazard ratio [HR] for death, 0.82; 95.798% CI, 0.68-0.99; P = .03) and 36.2 vs 31.3 months, respectively, in the overall population (HR, 0.84; 95% CI, 0.72-0.98; P = .03). Median progression-free survival for panitumumab vs bevacizumab was 13.1 vs 11.9 months, respectively, for those with left-sided tumors (HR, 1.00; 95% CI, 0.83-1.20) and 12.2 vs 11.4 months overall (HR, 1.05; 95% CI, 0.90-1.24). Response rates with panitumumab vs bevacizumab were 80.2% vs 68.6%, respectively, for left-sided tumors (difference, 11.2%; 95% CI, 4.4%-17.9%) and 74.9% vs 67.3% overall (difference, 7.7%; 95% CI, 1.5%-13.8%). Median duration of response with panitumumab vs bevacizumab was 13.1 vs 11.2 months for left-sided tumors (HR, 0.86; 95% CI, 0.70-1.10) and 11.9 vs 10.7 months overall (HR, 0.89; 95% CI, 0.74-1.06). Curative resection rates with panitumumab vs bevacizumab were 18.3% vs 11.6% for left-sided tumors; (difference, 6.6%; 95% CI, 1.0%-12.3%) and 16.5% vs 10.9% overall (difference, 5.6%; 95% CI, 1.0%-10.3%). Common treatment-emergent adverse events were acneiform rash (panitumumab: 74.8%; bevacizumab: 3.2%), peripheral sensory neuropathy (panitumumab: 70.8%; bevacizumab: 73.7%), and stomatitis (panitumumab: 61.6%; bevacizumab: 40.5%).
Conclusions and relevance: Among patients with RAS wild-type metastatic colorectal cancer, adding panitumumab, compared with bevacizumab, to standard first-line chemotherapy significantly improved overall survival in those with left-sided tumors and in the overall population.
Trial registration: ClinicalTrials.gov Identifier: NCT02394795.
Conflict of interest statement
Figures

References
-
- Gunawardene A, Desmond B, Shekouh A, Larsen P, Dennett E. Disease recurrence following surgery for colorectal cancer: five-year follow-up. N Z Med J. 2018;131(1469):51-58. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

