Clinical features and outcome of vertebral osteomyelitis after spinal injection: is it worth the price?
- PMID: 37071309
- PMCID: PMC10205873
- DOI: 10.1007/s15010-023-02024-9
Clinical features and outcome of vertebral osteomyelitis after spinal injection: is it worth the price?
Abstract
Purpose: Spinal injections are increasingly used for back pain treatment. Vertebral osteomyelitis (VO) after spinal injection (SIVO) is rare, but patient characteristics and outcome have not been well characterized. The aim of this study was to assess patient characteristics of SIVO in comparison to patients with native vertebral osteomyelitis (NVO) and to determine predictors for 1-year survival.
Methods: This is a single-center cohort study from a tertiary referral hospital. This is a retrospective analysis of Patients with VO who were prospectively enrolled into a spine registry from 2008 to 2019. Student's t-test, Kruskal-Wallis test or Chi-square test were applied for group comparisons. Survival analysis was performed using a log-rank test and a multivariable Cox regression model.
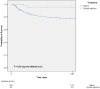
Results: 283 VO patients were enrolled in the study, of whom 44 (15.5%) had SIVO and 239 (84.5%) NVO. Patients with SIVO were significantly younger, had a lower Charlson comorbidity index and a shorter hospital stay compared to NVO. They also showed a higher rate of psoas abscesses and spinal empyema (38.6% [SIVO] vs. 20.9% [NVO]). Staphylococcus aureus (27%) and coagulase-negative staphylococci (CNS) (25%) were equally often detected in SIVO while S. aureus was more frequently than CNS in NVO (38.1% vs. 7.9%).Patients with SIVO (P = 0.04) had a higher 1-year survival rate (Fig. 1). After multivariate analysis, ASA score was associated with a lower 1-year survival in VO.
Conclusion: The results from this study emphasize unique clinical features of SIVO, which warrant that SIVO should be estimated as a separate entity of VO.
Keywords: Comorbidity; Psoas abscess; Spinal injection; Staphylococcus aureus; Survival; Vertebral osteomyelitis.
© 2023. The Author(s).
Conflict of interest statement
Each author certifies that there are no funding or commercial associations (consultancies, stock ownership, equity interest, patent/licensing arrangements, etc.) that might pose a conflict of interest in connection with the submitted article related to the author or any immediate family members except for NJ. NJ has received lecture fees from Gilead, Infectopharm, MSD, Bayer; Medacta and Labor Stein and travel grants from Pfizer, Gilead, Basilea, Correvio, Pfizer and Novartis and grants from an observational study from Infectopharm.
Figures

References
-
- Research C for DE and. FDA Drug Safety Communication: FDA requires label changes to warn of rare but serious neurologic problems after epidural corticosteroid injections for pain. FDA 2019.
MeSH terms
LinkOut - more resources
Full Text Sources

