WNK1 promotes water homeostasis by acting as a central osmolality sensor for arginine vasopressin release
- PMID: 37071482
- PMCID: PMC10231991
- DOI: 10.1172/JCI164222
WNK1 promotes water homeostasis by acting as a central osmolality sensor for arginine vasopressin release
Abstract
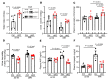
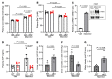
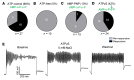
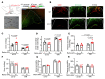
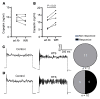
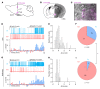
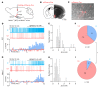
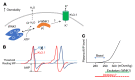
Maintaining internal osmolality constancy is essential for life. Release of arginine vasopressin (AVP) in response to hyperosmolality is critical. Current hypotheses for osmolality sensors in circumventricular organs (CVOs) of the brain focus on mechanosensitive membrane proteins. The present study demonstrated that intracellular protein kinase WNK1 was involved. Focusing on vascular-organ-of-lamina-terminalis (OVLT) nuclei, we showed that WNK1 kinase was activated by water restriction. Neuron-specific conditional KO (cKO) of Wnk1 caused polyuria with decreased urine osmolality that persisted in water restriction and blunted water restriction-induced AVP release. Wnk1 cKO also blunted mannitol-induced AVP release but had no effect on osmotic thirst response. The role of WNK1 in the osmosensory neurons in CVOs was supported by neuronal pathway tracing. Hyperosmolality-induced increases in action potential firing in OVLT neurons was blunted by Wnk1 deletion or pharmacological WNK inhibitors. Knockdown of Kv3.1 channel in OVLT by shRNA reproduced the phenotypes. Thus, WNK1 in osmosensory neurons in CVOs detects extracellular hypertonicity and mediates the increase in AVP release by activating Kv3.1 and increasing action potential firing from osmosensory neurons.
Keywords: Endocrinology; Epithelial transport of ions and water; Ion channels; Nephrology; Transport.
Conflict of interest statement
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

