Integrating Tumor-Intrinsic and Immunologic Factors to Identify Immunogenic Breast Cancers from a Low-Risk Cohort: Results from the Randomized SweBCG91RT Trial
- PMID: 37071498
- PMCID: PMC10150244
- DOI: 10.1158/1078-0432.CCR-22-2746
Integrating Tumor-Intrinsic and Immunologic Factors to Identify Immunogenic Breast Cancers from a Low-Risk Cohort: Results from the Randomized SweBCG91RT Trial
Abstract
Purpose: The local immune infiltrate's influence on tumor progression may be closely linked to tumor-intrinsic factors. The study aimed to investigate whether integrating immunologic and tumor-intrinsic factors can identify patients from a low-risk cohort who may be candidates for radiotherapy (RT) de-escalation.
Experimental design: The SweBCG91RT trial included 1,178 patients with stage I to IIA breast cancer, randomized to breast-conserving surgery with or without adjuvant RT, and followed for a median of 15.2 years. We trained two models designed to capture immunologic activity and immunomodulatory tumor-intrinsic qualities, respectively. We then analyzed if combining these two variables could further stratify tumors, allowing for identifying a subgroup where RT de-escalation is feasible, despite clinical indicators of a high risk of ipsilateral breast tumor recurrence (IBTR).
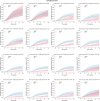
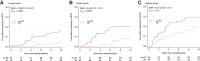
Results: The prognostic effect of the immunologic model could be predicted by the tumor-intrinsic model (Pinteraction = 0.01). By integrating measurements of the immunologic- and tumor-intrinsic models, patients who benefited from an active immune infiltrate could be identified. These patients benefited from standard RT (HR, 0.28; 95% CI, 0.09-0.85; P = 0.025) and had a 5.4% 10-year incidence of IBTR after irradiation despite high-risk genomic indicators and a low frequency of systemic therapy. In contrast, high-risk tumors without an immune infiltrate had a high 10-year incidence of IBTR despite RT treatment (19.5%; 95% CI, 12.2-30.3).
Conclusions: Integrating tumor-intrinsic and immunologic factors may identify immunogenic tumors in early-stage breast cancer populations dominated by ER-positive tumors. Patients who benefit from an activated immune infiltrate may be candidates for RT de-escalation.
©2023 The Authors; Published by the American Association for Cancer Research.
Figures
Comment in
- 1078-0432. doi: 10.1158/1078-0432.CCR-29-9-HI doi: 10.1158/1078-0432.CCR-29-9-HI
References
-
- Early Breast Cancer Trialists' Collaborative Group, Darby S, McGale P, Correa C, Taylor C, Arriagada R, et al. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet 2011;378:1707–16. - PMC - PubMed
-
- Speers C, Pierce LJ. Postoperative radiotherapy after breast-conserving surgery for early-stage breast cancer: a review. JAMA Oncol 2016;2:1075–82. - PubMed
-
- Schmid P, Cortes J, Pusztai L, McArthur H, Kümmel S, Bergh J, Denkert C, et al. Pembrolizumab for early triple-negative breast cancer. N Engl J Med 2020;382:810–21. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

