Activation of the PACAP/PAC1 Signaling Pathway Accelerates the Repair of Impaired Spatial Memory Caused by an Ultradian Light Cycle
- PMID: 37071544
- PMCID: PMC10123913
- DOI: 10.1177/17590914231169140
Activation of the PACAP/PAC1 Signaling Pathway Accelerates the Repair of Impaired Spatial Memory Caused by an Ultradian Light Cycle
Abstract
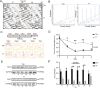
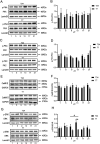
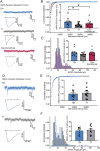
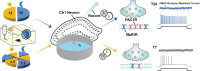
The mechanism of light-induced spatial memory deficits, as well as whether rhythmic expression of the pituitary adenylyl cyclase-activating polypeptides (PACAP)-PAC1 pathway influenced by light is related to this process, remains unclear. Here, we aimed to investigate the role of the PACAP-PAC1 pathway in light-mediated spatial memory deficits. Animals were first housed under a T24 cycle (12 h light:12 h dark), and then light conditions were transformed to a T7 cycle (3.5 h light:3.5 h dark) for at least 4 weeks. The spatial memory function was assessed using the Morris water maze (MWM). In line with behavioral studies, rhythmic expression of the PAC1 receptor and glutamate receptors in the hippocampal CA1 region was assessed by western blotting, and electrophysiology experiments were performed to determine the influence of the PACAP-PAC1 pathway on neuronal excitability and synaptic signaling transmission. Spatial memory was deficient after mice were exposed to the T7 light cycle. Rhythmic expression of the PAC1 receptor was dramatically decreased, and the excitability of CA1 pyramidal cells was decreased in T7 cycle-housed mice. Compensation with PACAP1-38, a PAC1 receptor agonist, helped T7 cycle-housed mouse CA1 pyramidal cells recover neuronal excitability to normal levels, and cannulas injected with PACAP1-38 shortened the time to find the platform in MWM. Importantly, the T7 cycle decreased the frequency of AMPA receptor-mediated excitatory postsynaptic currents. In conclusion, the PACAP-PAC1 pathway is an important protective factor modulating light-induced spatial memory function deficits, affecting CA1 pyramidal cell excitability and excitatory synaptic signaling transmission.
Keywords: CA1 pyramidal cell; Morris water maze; PACAP-PAC1 pathway; excitability; light; spatial memory.
Conflict of interest statement
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures


Similar articles
-
Modulation of AMPA receptor-mediated ion current by pituitary adenylate cyclase-activating polypeptide (PACAP) in CA1 pyramidal neurons from rat hippocampus.Hippocampus. 2009 Jan;19(1):99-109. doi: 10.1002/hipo.20488. Hippocampus. 2009. PMID: 18727050
-
PACAP-Induced PAC1 Receptor Internalization and Recruitment of Endosomal Signaling Regulate Cardiac Neuron Excitability.J Mol Neurosci. 2019 Jul;68(3):340-347. doi: 10.1007/s12031-018-1127-x. Epub 2018 Jul 27. J Mol Neurosci. 2019. PMID: 30054797 Free PMC article. Review.
-
Src family kinase inhibitors blunt PACAP-induced PAC1 receptor endocytosis, phosphorylation of ERK, and the increase in cardiac neuron excitability.Am J Physiol Cell Physiol. 2018 Feb 1;314(2):C233-C241. doi: 10.1152/ajpcell.00223.2017. Epub 2017 Nov 15. Am J Physiol Cell Physiol. 2018. PMID: 29141923 Free PMC article.
-
Inhibition of PACAP/PAC1/VPAC2 signaling impairs the consolidation of social recognition memory and nitric oxide prevents this deficit.Neurobiol Learn Mem. 2021 Apr;180:107423. doi: 10.1016/j.nlm.2021.107423. Epub 2021 Mar 9. Neurobiol Learn Mem. 2021. PMID: 33705861
-
PAC1 Receptor Internalization and Endosomal MEK/ERK Activation Is Essential for PACAP-Mediated Neuronal Excitability.J Mol Neurosci. 2021 Aug;71(8):1536-1542. doi: 10.1007/s12031-021-01821-x. Epub 2021 Mar 6. J Mol Neurosci. 2021. PMID: 33675454 Free PMC article. Review.
Cited by
-
Dexmedetomidine accelerates photoentrainment and affects sleep structure through the activation of SCNVIP neurons.Commun Biol. 2024 Dec 28;7(1):1707. doi: 10.1038/s42003-024-07430-9. Commun Biol. 2024. PMID: 39730868 Free PMC article.
References
-
- Ago, Y., Hiramatsu, N., Ishihama, T., Hazama, K., Hayata-Takano, A., Shibasaki, Y., Shintani, N., Hashimoto, H., Kawasaki, T., Onoe, H., Chaki, S., Nakazato, A., Baba, A., Takuma, K., & Matsuda, T. (2013). The selective metabotropic glutamate 2/3 receptor agonist MGS0028 reverses psychomotor abnormalities and recognition memory deficits in mice lacking the pituitary adenylate cyclase-activating polypeptide. Behav Pharmacol, 24(1), 74–77. 10.1097/FBP.0b013e32835cf3e5 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
