Dorfman pooling enhances SARS-CoV-2 large-scale community testing efficiency
- PMID: 37071604
- PMCID: PMC10112776
- DOI: 10.1371/journal.pgph.0001793
Dorfman pooling enhances SARS-CoV-2 large-scale community testing efficiency
Abstract
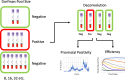
PCR-based analysis is the gold standard for detection of SARS-CoV-2 and was used broadly throughout the pandemic. However, heightened demand for testing put strain on diagnostic resources and the adequate amount of PCR-based testing required exceeded existing testing capacity. Pooled testing strategies presented an effective method to increase testing capacity by decreasing the number of tests and resources required for laboratory PCR analysis of SARS-CoV-2. We sought to conduct an analysis of SARS-CoV-2 pooling schemes to determine the sensitivity of various sized Dorfman pooling strategies and evaluate the utility of using such pooling strategies in diagnostic laboratory settings. Overall, a trend of decreasing sensitivity with larger pool sizes was observed, with modest sensitivity losses in the largest pools tested, and high sensitivity in all other pools. Efficiency data was then calculated to determine the optimal Dorfman pool sizes based on test positivity rate. This was correlated with current presumptive test positivity to maximize the number of tests saved, thereby increasing testing capacity and resource efficiency in the community setting. Dorfman pooling methods were evaluated and found to offer a high-throughput solution to SARS-CoV-2 clinical testing that improve resource efficiency in low-resource environments.
Copyright: © 2023 Burtniak et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
-
- Diagnostic Strategies for COVID-19 and other Coronaviruses. 2020. doi: 10.1007/978-981-15-6006-4 - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous
