Exclusive Human Milk Diet for Extremely Premature Infants: A Novel Fortification Strategy That Enhances the Bioactive Properties of Fresh, Frozen, and Pasteurized Milk Specimens
- PMID: 37071630
- PMCID: PMC10124176
- DOI: 10.1089/bfm.2022.0254
Exclusive Human Milk Diet for Extremely Premature Infants: A Novel Fortification Strategy That Enhances the Bioactive Properties of Fresh, Frozen, and Pasteurized Milk Specimens
Abstract
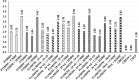
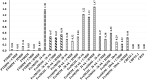
Background: Human milk (HM) fortification has been recommended for the nutritional optimization of very low-birthweight infants. This study analyzed the bioactive components of HM and evaluated fortification choices that could accentuate or attenuate the concentration of such components, with special reference to human milk-derived fortifier (HMDF) offered to extremely premature infants as an exclusive human milk diet. Materials and Methods: An observational feasibility study analyzed the biochemical and immunochemical characteristics of mothers' own milk (MOM), both fresh and frozen, and pasteurized banked donor human milk (DHM), each supplemented with either HMDF or cow's milk-derived fortifier (CMDF). Gestation-specific specimens were analyzed for macronutrients, pH, total solids, antioxidant activity (AA), α-lactalbumin, lactoferrin, lysozyme, and α- and β-caseins. Data were analyzed for variance applying general linear model and Tukey's test for pairwise comparison. Results: DHM exhibited significantly lower (p < 0.05) lactoferrin and α-lactalbumin concentrations than fresh and frozen MOM. HMDF reinstated lactoferrin and α-lactalbumin and exhibited higher protein, fat, and total solids (p < 0.05) in comparison to unfortified and CMDF-supplemented specimens. HMDF had the highest (p < 0.05) AA, suggesting the potential capability of HMDF to enhance oxidative scavenging. Conclusion: DHM, compared with MOM, has reduced bioactive properties, and CMDF conferred the least additional bioactive components. Reinstatement and further enhancement of bioactivity, which has been attenuated through pasteurization of DHM, is demonstrated through HMDF supplementation. Freshly expressed MOM fortified with HMDF and given early, enterally, and exclusively (3E) appears an optimal nutritional choice for extremely premature infants.
Keywords: breastfeeding; donor human milk; donor milk bank; exclusive human milk diet; human milk-derived fortifier; necrotizing enterocolitis; pasteurization.
Conflict of interest statement
The authors have declared their conflicts of interest through prescribed ICMJE forms. RKP has spoken at conferences hosted by Prolacta Bioscience, Nestle and Sanofi; was offered/received honorarium/attendance expenses.
Figures




Similar articles
-
Preterm Infants Fed Cow's Milk-Derived Fortifier Had Adverse Outcomes Despite a Base Diet of Only Mother's Own Milk.Breastfeed Med. 2020 May;15(5):297-303. doi: 10.1089/bfm.2019.0133. Epub 2020 Apr 2. Breastfeed Med. 2020. PMID: 32239968 Free PMC article.
-
Transient Hypoglycemia and Biochemical Differences in Infants Less Than 1,250 G at Birth Fed Human Milk with Human Milk-Derived Fortifier versus Cow Milk-Derived Fortifier.Am J Perinatol. 2024 May;41(S 01):e2824-e2831. doi: 10.1055/a-2164-7957. Epub 2023 Sep 1. Am J Perinatol. 2024. PMID: 37657486
-
Human Milk-Derived Fortifier as Rescue Therapy in Very Preterm Infants Intolerant to Cow's Milk-Derived Fortifier.Indian J Pediatr. 2022 Nov;89(11):1131-1133. doi: 10.1007/s12098-022-04309-7. Epub 2022 Jul 29. Indian J Pediatr. 2022. PMID: 35904719
-
Human milk is the feeding strategy to prevent necrotizing enterocolitis!Semin Perinatol. 2017 Feb;41(1):36-40. doi: 10.1053/j.semperi.2016.09.016. Epub 2016 Nov 8. Semin Perinatol. 2017. PMID: 27836421 Review.
-
Use of human milk and fortification in the NICU.J Perinatol. 2023 May;43(5):551-559. doi: 10.1038/s41372-022-01532-0. Epub 2022 Oct 18. J Perinatol. 2023. PMID: 36257977 Review.
Cited by
-
Neonatal bacteraemia in Ireland: A ten-year single-institution retrospective review.PLoS One. 2024 Aug 23;19(8):e0306855. doi: 10.1371/journal.pone.0306855. eCollection 2024. PLoS One. 2024. PMID: 39178209 Free PMC article.
-
Exclusive human milk diet is associated with lower risk of motor function impairment at three years of corrected age.J Perinatol. 2025 Apr 21. doi: 10.1038/s41372-025-02296-z. Online ahead of print. J Perinatol. 2025. PMID: 40259098
-
Operation Status of the Mutual Aid Human Milk Bank for Preterm Infants and Data Analysis [Letter].J Multidiscip Healthc. 2023 Nov 27;16:3661-3662. doi: 10.2147/JMDH.S450550. eCollection 2023. J Multidiscip Healthc. 2023. PMID: 38046051 Free PMC article. No abstract available.
References
-
- Eidelman AI. Breastfeeding and the use of human milk: An analysis of the American Academy of Pediatrics 2012 Breastfeeding Policy Statement. Breastfeeding Med 2012;7:323c4. - PubMed
-
- Committee on Nutrition, Section on Breastfeeding, Committee on Fetus and Newborn. Donor human milk for the high-risk infant: Preparation, safety, and usage options in the United States. Pediatrics 2017;139(1):3–4. - PubMed
-
- WHO. Donor human milk for low birth-weight infants. 2019. Available from: https://www.who.int/elena/titles/donormilk_infants/en (Last accessed: March 14, 2022).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
