Changes in eligibility for a subcutaneous cardioverter-defibrillator after implantation of a left ventricular assist device-A prospective analysis
- PMID: 37071637
- PMCID: PMC10112775
- DOI: 10.1371/journal.pone.0284419
Changes in eligibility for a subcutaneous cardioverter-defibrillator after implantation of a left ventricular assist device-A prospective analysis
Abstract
Background: The number of left ventricular assist devices (LVADs) implanted in patients with end-stage heart failure is increasing. In this patient cohort, subcutaneous implantable cardioverter defibrillators (S-ICDs) could be a promising alternative to transvenous ICDs due to lower infection rates and avoidance of venous access. However, eligibility for the S-ICD depends on ECG features that may be influenced by an LVAD. The aim of the present study was a prospective evaluation of S-ICD eligibility before and after LVAD implantation.
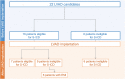
Methods: The study recruited all patients presenting at Hannover Medical School for LVAD implantation between 2016 and 2020. S-ICD eligibility was evaluated using the ECG-based and the device-based S-ICD screening test before and after LVAD implantation.
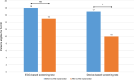
Results: Twenty-two patients (57.3 ± 8.7 years of age, 95.5% male) were included in the analysis. The most common underlying diseases were dilated cardiomyopathy (n = 16, 72.7%) and ischemic cardiomyopathy (n = 5, 22.7%). Before LVAD implantation 16 patients were found eligible for the S-ICD according to both screening tests (72.7%), but only 7 patients were eligible after LVAD, 31.8%; p = 0.05). Oversensing due to electromagnetic interference was observed in 6 patients (66.6%) found ineligible for S-ICD after LVAD implantation. A lower S wave amplitude in leads I (p = 0.009), II (p = 0.006) and aVF (p = 0.006) before LVAD implantation was associated with higher rate of S-ICD ineligibility after LVAD implantation.
Conclusion: LVAD implantation can impair S-ICD eligibility. Patients with lower S wave amplitude in leads I, II and aVF were more likely to be ineligible for S-ICD implantation after LVAD implantation. Thus, S-ICD therapy should be carefully considered in patients who are candidates for LVAD therapy.
Copyright: © 2023 Zormpas et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
I have read the journal’s policy and the authors of this manuscript have the following competing interests: CZ received travel grants and a fellowship grant from Biotronik and Medtronic. SH received a fellowship grant from Boston Scientific. JML received lecture honorary, travel grants and/or a fellowship grant from Biotronik, Boston Scientific, Medtronic and Zoll. CV received lecture honorary/advisory board fees from Biotronik, Medtronic, Abbott. DD received lecture honorary, travel grants and/or a fellowship grant from Abbott, Astra Zeneca, Bayer, Biotronik, Boehringer Ingelheim, Boston Scientific, Bristol Myers Squibb, Medtronic, Microport, Pfizer, Zoll. JE, HAKH and JDS do not report any financial disclosures. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures

Similar articles
-
Left ventricular assist device in the presence of subcutaneous implantable cardioverter defibrillator: Data from a multicenter experience.Int J Cardiol. 2024 Apr 1;400:131807. doi: 10.1016/j.ijcard.2024.131807. Epub 2024 Jan 23. Int J Cardiol. 2024. PMID: 38272130
-
Electromagnetic interference from left ventricular assist devices in patients with subcutaneous implantable cardioverter-defibrillators.J Cardiovasc Electrophysiol. 2020 May;31(5):1195-1201. doi: 10.1111/jce.14431. Epub 2020 Mar 9. J Cardiovasc Electrophysiol. 2020. PMID: 32128931 Review.
-
Eligibility for subcutaneous implantable cardioverter-defibrillator in patients with left ventricular assist device.J Interv Card Electrophysiol. 2021 Mar;60(2):303-311. doi: 10.1007/s10840-020-00810-1. Epub 2020 Jul 1. J Interv Card Electrophysiol. 2021. PMID: 32613315 Free PMC article.
-
Electrocardiographic changes after implantation of a left ventricular assist device - Potential implications for subcutaneous defibrillator therapy.J Electrocardiol. 2019 Jan-Feb;52:29-34. doi: 10.1016/j.jelectrocard.2018.11.002. Epub 2018 Nov 2. J Electrocardiol. 2019. PMID: 30476635
-
Wearable cardioverter defibrillator: bridging for implantable defibrillators in left ventricular assist device patients.Heart Fail Rev. 2021 Jul;26(4):763-765. doi: 10.1007/s10741-020-10064-8. Epub 2021 Jan 6. Heart Fail Rev. 2021. PMID: 33404997 Review.
Cited by
-
[Practical guidance for the implantation of non-transvenous ICD systems].Herzschrittmacherther Elektrophysiol. 2024 Sep;35(3):226-233. doi: 10.1007/s00399-024-01042-w. Epub 2024 Aug 21. Herzschrittmacherther Elektrophysiol. 2024. PMID: 39168897 Free PMC article. German.
References
-
- Zimpfer D, Fiane AE, Larbalestier R, Tsui S, Jansz P, Simon A, et al.. Long-Term Survival of Patients With Advanced Heart Failure Receiving an Left Ventricular Assist Device Intended as a Bridge to Transplantation. Circ Heart Fail. 2020;13: e006252. doi: 10.1161/CIRCHEARTFAILURE.119.006252 - DOI - PubMed
-
- Pignalosa L, Casula M, Rordorf R, Perna E, Baroni M, Garascia A, et al.. A multicentric observational study of patients affected by advanced heart failure with implantable cardioverter defibrillator and left ventricular assist devices. EP Europace. 2022;24: euac053.468. doi: 10.1093/europace/euac053.468 - DOI - PubMed
-
- Hohmann S, Heimeshoff J, Mueller-Leisse J, Hillmann H, Eiringhaus J, Zormpas C, et al.. Delayed detection programming significantly reduces inappropriate ICD therapies in patients with left-ventricular assist devices. EP Europace. 2022;24: euac053.457. doi: 10.1093/europace/euac053.457 - DOI
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

