Metabolic compatibility and the rarity of prokaryote endosymbioses
- PMID: 37071674
- PMCID: PMC10151612
- DOI: 10.1073/pnas.2206527120
Metabolic compatibility and the rarity of prokaryote endosymbioses
Abstract
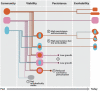
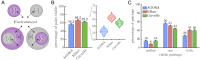
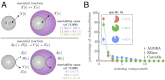
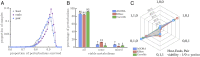
The evolution of the mitochondria was a significant event that gave rise to the eukaryotic lineage and most large complex life. Central to the origins of the mitochondria was an endosymbiosis between prokaryotes. Yet, despite the potential benefits that can stem from a prokaryotic endosymbiosis, their modern occurrence is exceptionally rare. While many factors may contribute to their rarity, we lack methods for estimating the extent to which they constrain the appearance of a prokaryotic endosymbiosis. Here, we address this knowledge gap by examining the role of metabolic compatibility between a prokaryotic host and endosymbiont. We use genome-scale metabolic flux models from three different collections (AGORA, KBase, and CarveMe) to assess the viability, fitness, and evolvability of potential prokaryotic endosymbioses. We find that while more than half of host-endosymbiont pairings are metabolically viable, the resulting endosymbioses have reduced growth rates compared to their ancestral metabolisms and are unlikely to gain mutations to overcome these fitness differences. In spite of these challenges, we do find that they may be more robust in the face of environmental perturbations at least in comparison with the ancestral host metabolism lineages. Our results provide a critical set of null models and expectations for understanding the forces that shape the structure of prokaryotic life.
Keywords: endosymbiosis; eukaryogenesis; evolution; metabolic model; prokaryote.
Conflict of interest statement
The authors declare no competing interest.
Figures

References
-
- Lane N., Serial endosymbiosis or singular event at the origin of eukaryotes? J. Theor. Biol. 434, 58–67 (2017). - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

