DNA hydrogels for bone regeneration
- PMID: 37071684
- PMCID: PMC10151614
- DOI: 10.1073/pnas.2220565120
DNA hydrogels for bone regeneration
Abstract
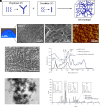
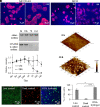
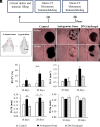
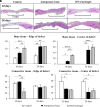
DNA-based biomaterials have been proposed for tissue engineering approaches due to their predictable assembly into complex morphologies and ease of functionalization. For bone tissue regeneration, the ability to bind Ca2+ and promote hydroxyapatite (HAP) growth along the DNA backbone combined with their degradation and release of extracellular phosphate, a known promoter of osteogenic differentiation, make DNA-based biomaterials unlike other currently used materials. However, their use as biodegradable scaffolds for bone repair remains scarce. Here, we describe the design and synthesis of DNA hydrogels, gels composed of DNA that swell in water, their interactions in vitro with the osteogenic cell lines MC3T3-E1 and mouse calvarial osteoblast, and their promotion of new bone formation in rat calvarial wounds. We found that DNA hydrogels can be readily synthesized at room temperature, and they promote HAP growth in vitro, as characterized by Fourier transform infrared spectroscopy, X-ray diffraction, scanning electron microscopy, atomic force microscopy, and transmission electron microscopy. Osteogenic cells remain viable when seeded on DNA hydrogels in vitro, as characterized by fluorescence microscopy. In vivo, DNA hydrogels promote the formation of new bone in rat calvarial critical size defects, as characterized by micro-computed tomography and histology. This study uses DNA hydrogels as a potential therapeutic biomaterial for regenerating lost bone.
Keywords: DNA hydrogels; DNA nanotechnology; biomineralization; bone regeneration.
Conflict of interest statement
The authors declare no competing interest.
Figures

References
-
- Seeman N. C., Sleiman H. F., DNA nanotechnology. Nat. Rev. Mater. 3, 17068 (2018).
-
- Hong F., Zhang F., Liu Y., Yan H., Origami D. N. A., Scaffolds for creating higher order structures. Chem. Rev. 117, 12584–12640 (2017). - PubMed
-
- Wang D., Liu P., Luo D., Putting DNA to work as generic polymeric materials. Angew. Chem. Int. Ed. 61, e202110666 (2022). - PubMed
-
- Carneiro K. M. M., Avakyan N., Sleiman H. F., Long-range assembly of DNA into nanofibers and highly ordered networks. Wires Nanomed. Nanobi. 5, 266–285 (2013). - PubMed
-
- Sethi S., Sugiyama H., Endo M., Biomimetic DNA nanotechnology to understand and control cellular responses. ChemBioChem 23, e202100446 (2022). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

