Characterization of Pairs of Toxic and Nontoxic Misfolded Protein Oligomers Elucidates the Structural Determinants of Oligomer Toxicity in Protein Misfolding Diseases
- PMID: 37071750
- PMCID: PMC10286310
- DOI: 10.1021/acs.accounts.3c00045
Characterization of Pairs of Toxic and Nontoxic Misfolded Protein Oligomers Elucidates the Structural Determinants of Oligomer Toxicity in Protein Misfolding Diseases
Abstract
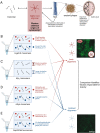
The aberrant misfolding and aggregation of peptides and proteins into amyloid aggregates occurs in over 50 largely incurable protein misfolding diseases. These pathologies include Alzheimer's and Parkinson's diseases, which are global medical emergencies owing to their prevalence in increasingly aging populations worldwide. Although the presence of mature amyloid aggregates is a hallmark of such neurodegenerative diseases, misfolded protein oligomers are increasingly recognized as of central importance in the pathogenesis of many of these maladies. These oligomers are small, diffusible species that can form as intermediates in the amyloid fibril formation process or be released by mature fibrils after they are formed. They have been closely associated with the induction of neuronal dysfunction and cell death. It has proven rather challenging to study these oligomeric species because of their short lifetimes, low concentrations, extensive structural heterogeneity, and challenges associated with producing stable, homogeneous, and reproducible populations. Despite these difficulties, investigators have developed protocols to produce kinetically, chemically, or structurally stabilized homogeneous populations of protein misfolded oligomers from several amyloidogenic peptides and proteins at experimentally ameneable concentrations. Furthermore, procedures have been established to produce morphologically similar but structurally distinct oligomers from the same protein sequence that are either toxic or nontoxic to cells. These tools offer unique opportunities to identify and investigate the structural determinants of oligomer toxicity by a close comparative inspection of their structures and the mechanisms of action through which they cause cell dysfunction.This Account reviews multidisciplinary results, including from our own groups, obtained by combining chemistry, physics, biochemistry, cell biology, and animal models for pairs of toxic and nontoxic oligomers. We describe oligomers comprised of the amyloid-β peptide, which underlie Alzheimer's disease, and α-synuclein, which are associated with Parkinson's disease and other related neurodegenerative pathologies, collectively known as synucleinopathies. Furthermore, we also discuss oligomers formed by the 91-residue N-terminal domain of [NiFe]-hydrogenase maturation factor from E. coli, which we use as a model non-disease-related protein, and by an amyloid stretch of Sup35 prion protein from yeast. These oligomeric pairs have become highly useful experimental tools for studying the molecular determinants of toxicity characteristic of protein misfolding diseases. Key properties have been identified that differentiate toxic from nontoxic oligomers in their ability to induce cellular dysfunction. These characteristics include solvent-exposed hydrophobic regions, interactions with membranes, insertion into lipid bilayers, and disruption of plasma membrane integrity. By using these properties, it has been possible to rationalize in model systems the responses to pairs of toxic and nontoxic oligomers. Collectively, these studies provide guidance for the development of efficacious therapeutic strategies to target rationally the cytotoxicity of misfolded protein oligomers in neurodegenerative conditions.
Conflict of interest statement
The authors declare the following competing financial interest(s): M.V. is a co-founder of Wren Therapeutics Limited, which is pursuing inhibitors of protein misfolding and aggregation.
Figures

Similar articles
-
Quantitative Measurement of the Affinity of Toxic and Nontoxic Misfolded Protein Oligomers for Lipid Bilayers and of its Modulation by Lipid Composition and Trodusquemine.ACS Chem Neurosci. 2021 Sep 1;12(17):3189-3202. doi: 10.1021/acschemneuro.1c00327. Epub 2021 Aug 12. ACS Chem Neurosci. 2021. PMID: 34382791 Free PMC article.
-
Structure of amyloid oligomers and their mechanisms of toxicities: Targeting amyloid oligomers using novel therapeutic approaches.Eur J Med Chem. 2016 May 23;114:41-58. doi: 10.1016/j.ejmech.2016.02.065. Epub 2016 Mar 2. Eur J Med Chem. 2016. PMID: 26974374 Review.
-
Camelid single-domain antibody fragments: Uses and prospects to investigate protein misfolding and aggregation, and to treat diseases associated with these phenomena.Biochimie. 2015 Apr;111:82-106. doi: 10.1016/j.biochi.2015.01.012. Epub 2015 Feb 3. Biochimie. 2015. PMID: 25656912 Review.
-
Elucidating the Role of Lipids in the Aggregation of Amyloidogenic Proteins.Acc Chem Res. 2023 Nov 7;56(21):2898-2906. doi: 10.1021/acs.accounts.3c00386. Epub 2023 Oct 12. Acc Chem Res. 2023. PMID: 37824095 Free PMC article.
-
Elucidating the Structures of Amyloid Oligomers with Macrocyclic β-Hairpin Peptides: Insights into Alzheimer's Disease and Other Amyloid Diseases.Acc Chem Res. 2018 Mar 20;51(3):706-718. doi: 10.1021/acs.accounts.7b00554. Epub 2018 Mar 6. Acc Chem Res. 2018. PMID: 29508987 Free PMC article. Review.
Cited by
-
Treatment of Alzheimer's Disease: Beyond Symptomatic Therapies.Int J Mol Sci. 2023 Sep 9;24(18):13900. doi: 10.3390/ijms241813900. Int J Mol Sci. 2023. PMID: 37762203 Free PMC article. Review.
-
From Molecular to Radionuclide and Pharmacological Aspects in Transthyretin Cardiac Amyloidosis.Int J Mol Sci. 2024 Dec 27;26(1):146. doi: 10.3390/ijms26010146. Int J Mol Sci. 2024. PMID: 39796004 Free PMC article. Review.
-
Thioflavin T─a Reporter of Microviscosity in Protein Aggregation Process: The Study Case of α-Synuclein.J Phys Chem Lett. 2024 Jun 27;15(25):6685-6690. doi: 10.1021/acs.jpclett.4c00699. Epub 2024 Jun 20. J Phys Chem Lett. 2024. PMID: 38899873 Free PMC article.
-
The Brain Toxin Cleansing of Sleep Achieved During Wakefulness.J Clin Med. 2025 Jan 31;14(3):926. doi: 10.3390/jcm14030926. J Clin Med. 2025. PMID: 39941597 Free PMC article.
-
Misfolded protein oligomers: mechanisms of formation, cytotoxic effects, and pharmacological approaches against protein misfolding diseases.Mol Neurodegener. 2024 Feb 20;19(1):20. doi: 10.1186/s13024-023-00651-2. Mol Neurodegener. 2024. PMID: 38378578 Free PMC article. Review.
References
-
- Cremades N.; Cohen S. I. A.; Deas E.; Abramov A. Y.; Chen A. Y.; Orte A.; Sandal M.; Clarke R. W.; Dunne P.; Aprile F. A.; Bertoncini C. W.; Wood N. W.; Knowles T. P. J.; Dobson C. M.; Klenerman D. Direct Observation of the Interconversion of Normal and Toxic Forms of α-Synuclein. Cell 2012, 149, 1048–1059. 10.1016/j.cell.2012.03.037. - DOI - PMC - PubMed
-
- Fusco G.; Chen S. W.; Williamson P. T. F.; Cascella R.; Perni M.; Jarvis J. A.; Cecchi C.; Vendruscolo M.; Chiti F.; Cremades N.; Ying L.; Dobson C. M.; Simone A. D. Structural Basis of Membrane Disruption and Cellular Toxicity by α-Synuclein Oligomers. Science 2017, 358, 1440–1443. 10.1126/science.aan6160. - DOI - PubMed
-
- Ladiwala A. R. A.; Litt J.; Kane R. S.; Aucoin D. S.; Smith S. O.; Ranjan S.; Davis J.; Nostrand W. E. V.; Tessier P. M. Conformational Differences between Two Amyloid β Oligomers of Similar Size and Dissimilar Toxicity. J. Biol. Chem. 2012, 287, 24765–24773. 10.1074/jbc.M111.329763. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

