Synthesis of 2-Cyanomethyl Indane Derivatives via Pd-Catalyzed Alkene Difunctionalization Reactions of Alkyl Nitriles
- PMID: 37071777
- PMCID: PMC10428494
- DOI: 10.1021/acs.orglett.3c00571
Synthesis of 2-Cyanomethyl Indane Derivatives via Pd-Catalyzed Alkene Difunctionalization Reactions of Alkyl Nitriles
Abstract
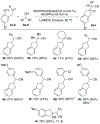
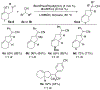
The synthesis of indanes bearing substituted cyanomethyl groups at C2 is achieved through Pd-catalyzed coupling reactions between 2-allylphenyl triflate derivatives and alkyl nitriles. Related partially saturated analogues were generated from analogous transformations of alkenyl triflates. The use of a preformed BrettPhosPd(allyl)(Cl) complex as a precatalyst was essential for the success of these reactions.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


Similar articles
-
Synthesis of Biindene Derivatives via Pd-Catalyzed Alkene Difunctionalization Reactions.Org Lett. 2024 May 24;26(20):4361-4364. doi: 10.1021/acs.orglett.4c01400. Epub 2024 May 15. Org Lett. 2024. PMID: 38747553
-
Pd-Catalyzed Alkene Difunctionalization Reactions of Enolates for the Synthesis of Substituted Bicyclic Cyclopentanes.Org Process Res Dev. 2019 Aug 16;23(8):1610-1630. doi: 10.1021/acs.oprd.9b00248. Epub 2019 Jul 30. Org Process Res Dev. 2019. PMID: 33597795 Free PMC article.
-
Pd-Catalyzed Alkene Difunctionalization Reactions of Malonate Nucleophiles: Synthesis of Substituted Cyclopentanes via Alkene Aryl-Alkylation and Akenyl-Alkylation.Org Lett. 2019 May 17;21(10):3813-3816. doi: 10.1021/acs.orglett.9b01258. Epub 2019 Apr 25. Org Lett. 2019. PMID: 31021646 Free PMC article.
-
Palladium-Catalyzed Synthesis of 1-Alkylidene-2-dialkylaminomethyl Cyclobutane Derivatives via Pd-Catalyzed Alkene Difunctionalization Reactions: Influence of Nucleophile and Water on the Reaction Mechanism.Org Lett. 2023 May 12;25(18):3245-3248. doi: 10.1021/acs.orglett.3c00954. Epub 2023 May 1. Org Lett. 2023. PMID: 37126729 Free PMC article.
-
Dehydrogenative Pd and Ni Catalysis for Total Synthesis.Acc Chem Res. 2021 Mar 2;54(5):1118-1130. doi: 10.1021/acs.accounts.0c00787. Epub 2021 Feb 16. Acc Chem Res. 2021. PMID: 33592147 Free PMC article. Review.
References
-
- White DR; Hinds EM; Bornowski EC; Wolfe JP “Pd-Catalyzed Alkene Difunctionalization Reactions of Malonate Nucleophiles. Synthesis of Substituted Cyclopentanes via Alkene Aryl-Alkylation and Alkenyl-Alkylation” Org. Lett 2019, 21, 3813. - PMC - PubMed
- Bornowski EC; Hinds EM; White DR; Nakamura Y; Wolfe JP “ Pd-Catalyzed Alkene Difunctionalization Reactions of Enolates for the Synthesis of Substituted Bicyclic Cyclopentanes” Org. Proc. Res. Dev 2019, 33, 1610. - PMC - PubMed
-
-
For related transformations of amine, alcohol, or indole nucleophiles, see:
- White DR; Hutt JT; Wolfe JP “Asymmetric Pd-Catalyzed Alkene Carboamination Reactions for the Synthesis of 2-Aminoindane Derivatives” J. Am. Chem. Soc 2015, 137, 11246. - PMC - PubMed
- White DR; Wolfe JP “Stereocontrolled Synthesis of Amino-Substituted Carbocycles via Pd-Catalyzed Alkene Carboamination Reactions” Chem. Eur. J 2017, 23, 5419. - PubMed
- White DR; Herman MI; Wolfe JP “Palladium-Catalyzed Alkene Carboalkoxylation Reactions of Phenols and Alcohols for the Synthesis of Carbocycles” Org. Lett 2017, 19, 4311. - PubMed
- Kirsch JK; Manske JL; Wolfe JP “Pd-Catalyzed Alkene Carboheteroarylation Reactions for the Synthesis of 3-Cyclopentylindole Derivatives” J. Org. Chem 2018, 83, 13568. - PMC - PubMed
-
-
- DeAngelis AJ; Gildner PJ; Chow R; Colacot TJ “Generating Active ‘L-Pd(0)’ via Neutral or Cationic π-Allylpalladium Complexes Featuring Biaryl/Bipyrazolylphospines: Synthetic, Mechanistic, and Structure-Activity Studies in Challenging Cross-Coupling Reactions” J. Org. Chem 2015, 80, 6794. - PubMed
-
- Orlandi M; Brenna D; Harms R; Jost S; Benaglia M “Recent Developments in the Reduction of Aromatic and Aliphatic Nitro Compounds to Amines” Org. Proc. Res. Dev 2018, 22, 430–445.
- Wang M-X; “Enantioselective Biotransformations of Nitriles in Organic Synthesis” Acc. Chem. Res 2015, 48, 602. - PubMed
- Fleming FF; Zhang Z “Cyclic Nitriles: Tactical Advantages in Synthesis” Tetrahedron 2005, 61, 747.
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

