Linearization of the Brevicidine and Laterocidine Lipopeptides Yields Analogues That Retain Full Antibacterial Activity
- PMID: 37071814
- PMCID: PMC10150354
- DOI: 10.1021/acs.jmedchem.3c00308
Linearization of the Brevicidine and Laterocidine Lipopeptides Yields Analogues That Retain Full Antibacterial Activity
Abstract
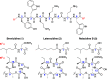
Brevicidine and laterocidine are macrocyclic lipodepsipeptides with selective activity against Gram-negative bacteria, including colistin-resistant strains. Previously, the macrocyclic core of these peptides was thought essential for antibacterial activity. In this study, we show that C-terminal amidation of linear brevicidine and laterocidine scaffolds, and substitution of the native Thr9, yields linear analogues that retain the potent antibacterial activity and low hemolysis of the parent compounds. Furthermore, an alanine scan of both peptides revealed that the aromatic and basic amino acids within the common central scaffold are essential for antibacterial activity. This linearization strategy for modification of cyclic peptides is a highly effective way to reduce the time and cost of peptide synthesis and may be applicable to other non-ribosomal antibacterial peptides.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

Similar articles
-
The Total Chemical Synthesis and Biological Evaluation of the Cationic Antimicrobial Peptides, Laterocidine and Brevicidine.J Nat Prod. 2021 Aug 27;84(8):2165-2174. doi: 10.1021/acs.jnatprod.1c00222. Epub 2021 Aug 2. J Nat Prod. 2021. PMID: 34338512
-
Synthetic studies with the brevicidine and laterocidine lipopeptide antibiotics including analogues with enhanced properties and in vivo efficacy.Chem Sci. 2022 Feb 23;13(12):3563-3570. doi: 10.1039/d2sc00143h. eCollection 2022 Mar 24. Chem Sci. 2022. PMID: 35432860 Free PMC article.
-
Synthesis, Structure-Activity Relationship Study, Bioactivity, and Nephrotoxicity Evaluation of the Proposed Structure of the Cyclic Lipodepsipeptide Brevicidine B.J Nat Prod. 2024 Apr 26;87(4):764-773. doi: 10.1021/acs.jnatprod.3c00876. Epub 2024 Feb 29. J Nat Prod. 2024. PMID: 38423998
-
Gram-negative bacilli-derived peptide antibiotics developed since 2000.Biotechnol Lett. 2018 Oct;40(9-10):1271-1287. doi: 10.1007/s10529-018-2589-1. Epub 2018 Jul 2. Biotechnol Lett. 2018. PMID: 29968134 Review.
-
Linear Analogues of the Lipopeptide Battacin With Potent In Vitro Activity Against S. aureus.Adv Protein Chem Struct Biol. 2018;112:385-394. doi: 10.1016/bs.apcsb.2018.03.004. Epub 2018 Apr 6. Adv Protein Chem Struct Biol. 2018. PMID: 29680242 Review.
Cited by
-
A novel approach for the synthesis of the cyclic lipopeptide globomycin.RSC Med Chem. 2024 Oct 21;16(1):373-8. doi: 10.1039/d4md00685b. Online ahead of print. RSC Med Chem. 2024. PMID: 39493230 Free PMC article.
-
Exploring Structure-Activity Relationships and Modes of Action of Laterocidine.ACS Cent Sci. 2024 Aug 6;10(9):1703-1717. doi: 10.1021/acscentsci.4c00776. eCollection 2024 Sep 25. ACS Cent Sci. 2024. PMID: 39345814 Free PMC article.
-
Antimicrobial Peptide-Peptoid Macrocycles from the Polymyxin B2 Chemical Space.Angew Chem Int Ed Engl. 2025 Jun 2;64(23):e202501299. doi: 10.1002/anie.202501299. Epub 2025 Apr 3. Angew Chem Int Ed Engl. 2025. PMID: 40138381 Free PMC article.
-
Design and Production of Geranylated Cyclic Peptides by the RiPP Enzymes SyncM and PirF.Biomacromolecules. 2025 May 12;26(5):3186-3199. doi: 10.1021/acs.biomac.5c00260. Epub 2025 Apr 6. Biomacromolecules. 2025. PMID: 40189806 Free PMC article.
-
Structure-Activity Study of the Antimicrobial Lipopeptide Humimycin A and Screening Against Multidrug-Resistant Staphylococcus aureus.Antibiotics (Basel). 2025 Apr 5;14(4):385. doi: 10.3390/antibiotics14040385. Antibiotics (Basel). 2025. PMID: 40298539 Free PMC article.
References
-
- O’Neill J.Review on Antibacterial Resistance. 2014.
-
- Koo H. B.; Seo J. Antimicrobial peptides under clinical investigation. Pept. Sci. 2019, 111, e2412210.1002/pep2.24122. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

