Design and 3D Printing of Personalized Hybrid and Gradient Structures for Critical Size Bone Defects
- PMID: 37071829
- PMCID: PMC10189796
- DOI: 10.1021/acsabm.3c00107
Design and 3D Printing of Personalized Hybrid and Gradient Structures for Critical Size Bone Defects
Abstract
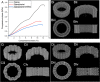
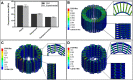
Treating critical-size bone defects with autografts, allografts, or standardized implants is challenging since the healing of the defect area necessitates patient-specific grafts with mechanically and physiologically relevant structures. Three-dimensional (3D) printing using computer-aided design (CAD) is a promising approach for bone tissue engineering applications by producing constructs with customized designs and biomechanical compositions. In this study, we propose 3D printing of personalized and implantable hybrid active scaffolds with a unique architecture and biomaterial composition for critical-size bone defects. The proposed 3D hybrid construct was designed to have a gradient cell-laden poly(ethylene glycol) (PEG) hydrogel, which was surrounded by a porous polycaprolactone (PCL) cage structure to recapitulate the anatomical structure of the defective area. The optimized PCL cage design not only provides improved mechanical properties but also allows the diffusion of nutrients and medium through the scaffold. Three different designs including zigzag, zigzag/spiral, and zigzag/spiral with shifting the zigzag layers were evaluated to find an optimal architecture from a mechanical point of view and permeability that can provide the necessary mechanical strength and oxygen/nutrient diffusion, respectively. Mechanical properties were investigated experimentally and analytically using finite element analysis (FEA), and computational fluid dynamics (CFD) simulation was used to determine the permeability of the structures. A hybrid scaffold was fabricated via 3D printing of the PCL cage structure and a PEG-based bioink comprising a varying number of human bone marrow mesenchymal stem cells (hBMSCs). The gradient bioink was deposited inside the PCL cage through a microcapillary extrusion to generate a mineralized gradient structure. The zigzag/spiral design for the PCL cage was found to be mechanically strong with sufficient and optimum nutrient/gas axial and radial diffusion while the PEG-based hydrogel provided a biocompatible environment for hBMSC viability, differentiation, and mineralization. This study promises the production of personalized constructs for critical-size bone defects by printing different biomaterials and gradient cells with a hybrid design depending on the need for a donor site for implantation.
Keywords: 3D printing; gradient structures; hybrid printing; large bone defects; personalized scaffolds.
Conflict of interest statement
The authors declare no competing financial interest.
Figures







Similar articles
-
Hybrid biofabrication of 3D osteoconductive constructs comprising Mg-based nanocomposites and cell-laden bioinks for bone repair.Bone. 2022 Jan;154:116198. doi: 10.1016/j.bone.2021.116198. Epub 2021 Sep 15. Bone. 2022. PMID: 34534709
-
Low temperature hybrid 3D printing of hierarchically porous bone tissue engineering scaffolds within situdelivery of osteogenic peptide and mesenchymal stem cells.Biofabrication. 2022 Aug 11;14(4). doi: 10.1088/1758-5090/ac84b0. Biofabrication. 2022. PMID: 35896092
-
Evaluation of the Usability of a Low-Cost 3D Printer in a Tissue Engineering Approach for External Ear Reconstruction.Int J Mol Sci. 2021 Oct 28;22(21):11667. doi: 10.3390/ijms222111667. Int J Mol Sci. 2021. PMID: 34769096 Free PMC article.
-
Advances in 3D printing of composite scaffolds for the repairment of bone tissue associated defects.Biotechnol Prog. 2022 May;38(3):e3234. doi: 10.1002/btpr.3234. Epub 2022 Feb 3. Biotechnol Prog. 2022. PMID: 35037419 Review.
-
Three-dimensional (3D) printed scaffold and material selection for bone repair.Acta Biomater. 2019 Jan 15;84:16-33. doi: 10.1016/j.actbio.2018.11.039. Epub 2018 Nov 24. Acta Biomater. 2019. PMID: 30481607 Review.
Cited by
-
Enhancing bone regeneration through 3D printed biphasic calcium phosphate scaffolds featuring graded pore sizes.Bioact Mater. 2024 Dec 9;46:21-36. doi: 10.1016/j.bioactmat.2024.11.024. eCollection 2025 Apr. Bioact Mater. 2024. PMID: 39734570 Free PMC article.
-
Advancements in hydrogel design for articular cartilage regeneration: A comprehensive review.Bioact Mater. 2024 Sep 14;43:1-31. doi: 10.1016/j.bioactmat.2024.09.005. eCollection 2025 Jan. Bioact Mater. 2024. PMID: 39318636 Free PMC article. Review.
-
3D bioprinted ferret mesenchymal stem cell-laden cartilage grafts for laryngotracheal reconstruction in a ferret surgical model.Biomater Sci. 2025 Feb 25;13(5):1304-1322. doi: 10.1039/d4bm01251h. Biomater Sci. 2025. PMID: 39886992 Free PMC article.
-
"Metal-bone" scaffold for accelerated peri-implant endosseous healing.Front Bioeng Biotechnol. 2024 Jan 10;11:1334072. doi: 10.3389/fbioe.2023.1334072. eCollection 2023. Front Bioeng Biotechnol. 2024. PMID: 38268934 Free PMC article.
-
Shape Memory PLA/TPU Blend Using High-Speed Thermo-Kinetic Mixing.ACS Omega. 2024 Dec 27;10(1):193-206. doi: 10.1021/acsomega.4c04338. eCollection 2025 Jan 14. ACS Omega. 2024. PMID: 39829531 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

