Systematic Review and Meta-analysis: The Association Between Serum Ustekinumab Trough Concentrations and Treatment Response in Inflammatory Bowel Disease
- PMID: 37071852
- PMCID: PMC10988107
- DOI: 10.1093/ibd/izad065
Systematic Review and Meta-analysis: The Association Between Serum Ustekinumab Trough Concentrations and Treatment Response in Inflammatory Bowel Disease
Abstract
Background: Optimizing therapy and monitoring response are integral aspects of inflammatory bowel disease treatment. We conducted a systematic review and meta-analysis to determine whether serum ustekinumab trough concentrations during maintenance therapy were associated with ustekinumab treatment response in patients with inflammatory bowel disease.
Methods: A systematic review was performed to March 21, 2022, to identify studies using MEDLINE, EMBASE, and the Cochrane library. We included studies that reported the association between serum ustekinumab trough concentrations with clinical or endoscopic remission. Outcome measures were combined across studies using the random-effects model with an odds ratio (OR) for binary outcomes of endoscopic and clinical remission.
Results: We identified 14 observational studies that were included in the analysis for clinical remission (919 patients, 63% with Crohn's disease) or endoscopic remission (290 patients, all with Crohn's disease). Median ustekinumab trough concentrations were higher amongst individuals achieving clinical remission compared with those not achieving remission (mean difference, 1.6 ug/mL; 95% confidence interval [CI], 0.21-3.01 ug/mL). Furthermore, individuals with median serum trough concentration in the fourth quartile were significantly more likely to achieve clinical (OR, 3.61; 95% CI, 2.11-6.20) but not endoscopic remission (OR, 4.67; 95% CI, 0.86-25.19) compared with those with first quartile median trough concentrations.
Conclusion: Based on the results of this meta-analysis primarily relating to patients with Crohn's disease on maintenance ustekinumab treatment, it appears that there is an association between higher ustekinumab trough concentration and clinical outcomes. Prospective studies are required to determine whether proactive dose adjustments of ustekinumab therapy provides additional clinical benefit.
Keywords: Crohn’s disease; biologics; monoclonal antibodies; therapeutic drug monitoring; ulcerative colitis.
Plain language summary
This meta-analysis of 14 observational studies found an association between better clinical outcomes and higher trough ustekinumab levels for maintenance treatment in inflammatory bowel disease.
© 2023 Crohn’s & Colitis Foundation. Published by Oxford University Press on behalf of Crohn’s & Colitis Foundation.
Conflict of interest statement
A.V., V.T., M.B., M.H.: none to declare
L.R.: Advisory boards: Janssens Pharmaceuticals, Fresenius Kabi
D.B.: Consulting: Janssens Pharmaceuticals
E.V.L.: Consulting for AbbVie, Amgen, Arena, Boehringer Ingelheim, Bristol-Myers Squibb, CALIBR, Celgene, Celltrion Healthcare, Eli Lilly, Fresenius Kabi, Genentech, Gilead, GlaxoSmithKline, Gossamer Bio, Iterative Scopes, Janssen, Morphic, Ono Pharma, Pfizer, Protagonist, Sun Pharma, Surrozen, Sun Pharma, Takeda, UCB; research support from AbbVie, Bristol-Myers Squibb, Celgene/Receptos, Genentech, Gilead, Gossamer Bio, Janssen, Pfizer, Takeda, Theravance, UCB.
Figures
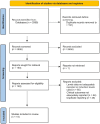



References
-
- Vande Casteele N, Ferrante M, Van Assche G, et al. Trough concentrations of infliximab guide dosing for patients with inflammatory bowel disease. Gastroenterology. 2015;148(7):1320-9.e3. - PubMed
-
- Feagan BG, Sandborn WJ, Gasink C, et al. ; UNITI–IM-UNITI Study Group. Ustekinumab as induction and maintenance therapy for Crohn’s disease. N Engl J Med. 2016;375(20):1946-1960. - PubMed
-
- Adedokun OJ, Xu Z, Gasink C, et al. Pharmacokinetics and exposure response relationships of ustekinumab in patients with Crohn’s disease. Gastroenterology. 2018;154(6):1660-1671. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

