Dynamic changes in P300 enhancers and enhancer-promoter contacts control mouse cardiomyocyte maturation
- PMID: 37071996
- PMCID: PMC10231645
- DOI: 10.1016/j.devcel.2023.03.020
Dynamic changes in P300 enhancers and enhancer-promoter contacts control mouse cardiomyocyte maturation
Abstract
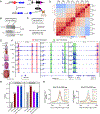
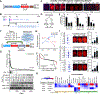
Cardiomyocyte differentiation continues throughout murine gestation and into the postnatal period, driven by temporally regulated expression changes in the transcriptome. The mechanisms that regulate these developmental changes remain incompletely defined. Here, we used cardiomyocyte-specific ChIP-seq of the activate enhancer marker P300 to identify 54,920 cardiomyocyte enhancers at seven stages of murine heart development. These data were matched to cardiomyocyte gene expression profiles at the same stages and to Hi-C and H3K27ac HiChIP chromatin conformation data at fetal, neonatal, and adult stages. Regions with dynamic P300 occupancy exhibited developmentally regulated enhancer activity, as measured by massively parallel reporter assays in cardiomyocytes in vivo, and identified key transcription factor-binding motifs. These dynamic enhancers interacted with temporal changes of the 3D genome architecture to specify developmentally regulated cardiomyocyte gene expressions. Our work provides a 3D genome-mediated enhancer activity landscape of murine cardiomyocyte development.
Keywords: Hi-C; HiChIP; cardiomyocyte maturation; enhancer; massively parallel reporter assay; nuclear receptor.
Copyright © 2023 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures





References
-
- Schoenfelder S, and Fraser P. (2019). Long-range enhancer–promoter contacts in gene expression control. Nat. Rev. Genet. 20, 437–455. - PubMed
-
- Stadhouders R, Filion GJ, and Graf T. (2019). Transcription factors and 3D genome conformation in cell-fate decisions. Nature 569, 345–354. - PubMed
-
- Zheng H, and Xie W. (2019). The role of 3D genome organization in development and cell differentiation. Nat. Rev. Mol. Cell Biol. 20, 535–550. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

