Epidemiology of extensively drug-resistant tuberculosis among patients with multidrug-resistant tuberculosis: A systematic review and meta-analysis
- PMID: 37072053
- PMCID: PMC10302157
- DOI: 10.1016/j.ijid.2023.04.392
Epidemiology of extensively drug-resistant tuberculosis among patients with multidrug-resistant tuberculosis: A systematic review and meta-analysis
Abstract
Objectives: To estimate the pooled proportion of extensively drug-resistant tuberculosis (XDR-TB) and pre-extensively drug-resistant tuberculosis (pre-XDR-TB) in patients with multidrug-resistant TB (MDR-TB).
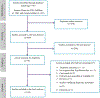
Methods: We systematically searched articles from electronic databases: MEDLINE (PubMed), ScienceDirect, and Google Scholar. We also searched gray literature from the different literature sources main outcome of the review was either XDR-TB or pre-XDR-TB in patients with MDR-TB. We used the random-effects model, considering the substantial heterogeneity among studies. Heterogeneity was assessed by subgroup analyses. STATA version 14 was used for analysis.
Results: A total of 64 studies that reported on 12,711 patients with MDR-TB from 22 countries were retrieved. The pooled proportion of pre-XDR-TB was 26% (95% confidence interval [CI]: 22-31%), whereas XDR-TB in MDR-TB cases was 9% (95% CI: 7-11%) in patients treated for MDR-TB. The pooled proportion of resistance to fluoroquinolones was 27% (95% CI: 22-33%) and second-line injectable drugs was 11% (95% CI: 9-13%). Whereas the pooled resistance proportions to bedaquiline, clofazimine, delamanid, and linezolid were 5% (95% CI: 1-8%), 4% (95% CI: 0-10%), 5% (95% CI; 2-8%), and 4% (95% CI: 2-10%), respectively.
Conclusion: The burden of pre-XDR-TB and XDR-TB in MDR-TB were considerable. The high burdens of pre-XDR-TB and XDR-TB in patients treated for MDR-TB suggests the need to strengthen TB programs and drug resistance surveillance.
Keywords: Extensively drug-resistant tuberculosis; Multidrug-resistant tuberculosis; Pre-extensively drug-resistant tuberculosis.
Copyright © 2023 The Author(s). Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of competing interests The authors have no competing interests to declare.
Figures






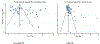
References
-
- World Health Organization. Global Tubeclosis report 2020.
-
- World Health Organization Global Tubeclosis report. Geneva: World Health Organization; 2022.
-
- World Health Organization Global tuberculosis report. Geneva: World Health Organization; 2019.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

