Prediction of Post-Acute-Sequelae of COVID-19 by Cargo Protein Biomarkers of Blood Total Extracellular Vesicles in Acute COVID-19
- PMID: 37072092
- PMCID: PMC10106499
- DOI: 10.1016/j.amjmed.2023.03.026
Prediction of Post-Acute-Sequelae of COVID-19 by Cargo Protein Biomarkers of Blood Total Extracellular Vesicles in Acute COVID-19
Abstract
Background: SARS-CoV-2 invades mitochondria of infected cells resulting in disordered metabolism, mitophagy, and abnormal levels of mitochondrial proteins in extracellular vesicles. Blood extracellular vesicle SARS-CoV-2 proteins and mitochondrial proteins were quantified in COVID-19 to assess possible roles as biomarkers.
Methods: Total extracellular vesicles were precipitated from blood of age- and gender-matched participants with no infection (n=10), acute COVID-19 (n=16), post-acute sequelae of COVID-19 (PASC or long COVID) (n=30), or post-acute COVID without PASC (n=8) and their extracted proteins quantified by enzyme-linked immunosorbent assays (ELISAs).
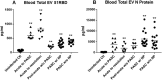
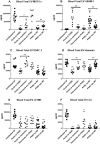
Results: Total extracellular vesicle levels of S1 (receptor-binding domain [RBD]) protein were significantly higher in acute infections than in uninfected controls, post-acute infection without PASC, and PASC. Total extracellular vesicle levels of nucleocapsid (N) protein were significantly higher in PASC than in uninfected controls, acute infections, and post-acute infection without PASC. Neither acute levels of S1(RBD) or N proteins predicted progression to PASC. Levels of neither SARS-CoV-2 protein in established PASC correlated with neuropsychiatric manifestations. Significant decreases in total extracellular vesicle levels of the mitochondrial proteins MOTS-c, VDAC-1, and humanin, and elevations of levels of SARM-1 were observed in acutely infected patients who would develop PASC. Significant decreases in total extracellular vesicle levels of MOTS-c and humanin, but not VDAC-1, and elevations of total extracellular vesicle levels of SARM-1 were characteristic of PASC patients with neuropsychiatric manifestations.
Conclusions: Total extracellular vesicle levels of SARS-CoV-2 proteins in COVID-19 indicate intracellular presence of SARS-CoV-2. Abnormal total extracellular vesicles levels of mitochondrial proteins in acute infections predict a high risk of PASC and later in established PASC are indicative of neuropsychiatric manifestations.
Keywords: Long COVID; Mitochondria; Neuropsychiatric effects of COVID-19; SARS-CoV-2 proteins S1 and N.
Copyright © 2023 The Authors. Published by Elsevier Inc. All rights reserved.
Figures
Similar articles
-
SARS-CoV-2 and Mitochondrial Proteins in Neural-Derived Exosomes of COVID-19.Ann Neurol. 2022 Jun;91(6):772-781. doi: 10.1002/ana.26350. Epub 2022 Mar 30. Ann Neurol. 2022. PMID: 35285072 Free PMC article.
-
Persistent circulation of soluble and extracellular vesicle-linked Spike protein in individuals with postacute sequelae of COVID-19.J Med Virol. 2023 Feb;95(2):e28568. doi: 10.1002/jmv.28568. J Med Virol. 2023. PMID: 36756925 Free PMC article.
-
A longitudinal SARS-CoV-2 biorepository for COVID-19 survivors with and without post-acute sequelae.BMC Infect Dis. 2021 Jul 13;21(1):677. doi: 10.1186/s12879-021-06359-2. BMC Infect Dis. 2021. PMID: 34256735 Free PMC article.
-
Long COVID or Post-Acute Sequelae of SARS-CoV-2 Infection (PASC) and the Urgent Need to Identify Diagnostic Biomarkers and Risk Factors.Med Sci Monit. 2024 Sep 18;30:e946512. doi: 10.12659/MSM.946512. Med Sci Monit. 2024. PMID: 39289865 Free PMC article. Review.
-
Pathogenic mechanisms of post-acute sequelae of SARS-CoV-2 infection (PASC).Elife. 2023 Mar 22;12:e86002. doi: 10.7554/eLife.86002. Elife. 2023. PMID: 36947108 Free PMC article. Review.
Cited by
-
Understanding Post-COVID-19: Mechanisms, Neurological Complications, Current Treatments, and Emerging Therapies.Int J Gen Med. 2024 Dec 17;17:6303-6321. doi: 10.2147/IJGM.S499905. eCollection 2024. Int J Gen Med. 2024. PMID: 39717071 Free PMC article.
-
Gut Microbiota and Mitochondria: Health and Pathophysiological Aspects of Long COVID.Int J Mol Sci. 2023 Dec 6;24(24):17198. doi: 10.3390/ijms242417198. Int J Mol Sci. 2023. PMID: 38139027 Free PMC article. Review.
-
Exploring the Pathophysiology of Long COVID: The Central Role of Low-Grade Inflammation and Multisystem Involvement.Int J Mol Sci. 2024 Jun 9;25(12):6389. doi: 10.3390/ijms25126389. Int J Mol Sci. 2024. PMID: 38928096 Free PMC article. Review.
-
Mitochondria in COVID-19: from cellular and molecular perspective.Front Physiol. 2024 Jun 21;15:1406635. doi: 10.3389/fphys.2024.1406635. eCollection 2024. Front Physiol. 2024. PMID: 38974521 Free PMC article. Review.
-
Extracellular vesicles: translational research and applications in neurology.Nat Rev Neurol. 2025 May;21(5):265-282. doi: 10.1038/s41582-025-01080-z. Epub 2025 Apr 3. Nat Rev Neurol. 2025. PMID: 40181198 Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

