The pro-inflammatory response to influenza A virus infection is fueled by endothelial cells
- PMID: 37072183
- PMCID: PMC10114347
- DOI: 10.26508/lsa.202201837
The pro-inflammatory response to influenza A virus infection is fueled by endothelial cells
Abstract
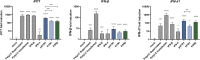
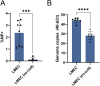
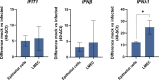
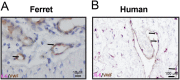
Morbidity and mortality from influenza are associated with high levels of systemic inflammation. Endothelial cells play a key role in systemic inflammatory responses during severe influenza A virus (IAV) infections, despite being rarely infected in humans. How endothelial cells contribute to systemic inflammatory responses is unclear. Here, we developed a transwell system in which airway organoid-derived differentiated human lung epithelial cells were co-cultured with primary human lung microvascular endothelial cells (LMECs). We compared the susceptibility of LMECs to pandemic H1N1 virus and recent seasonal H1N1 and H3N2 viruses and assessed the associated pro-inflammatory responses. Despite the detection of IAV nucleoprotein in LMEC mono-cultures, there was no evidence for productive infection. In epithelial-endothelial co-cultures, abundant IAV infection of epithelial cells resulted in the breakdown of the epithelial barrier, but infection of LMECs was rarely detected. We observed a significantly higher secretion of pro-inflammatory cytokines in LMECs when co-cultured with IAV-infected epithelial cells than LMEC mono-cultures exposed to IAV. Taken together, our data show that LMECs are abortively infected by IAV but can fuel the inflammatory response.
© 2023 Bauer et al.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures







Similar articles
-
Membrane-Tethered Mucin 1 Is Stimulated by Interferon and Virus Infection in Multiple Cell Types and Inhibits Influenza A Virus Infection in Human Airway Epithelium.mBio. 2022 Aug 30;13(4):e0105522. doi: 10.1128/mbio.01055-22. Epub 2022 Jun 14. mBio. 2022. PMID: 35699372 Free PMC article.
-
Differential Modulation of Innate Immune Responses in Human Primary Cells by Influenza A Viruses Carrying Human or Avian Nonstructural Protein 1.J Virol. 2019 Dec 12;94(1):e00999-19. doi: 10.1128/JVI.00999-19. Print 2019 Dec 12. J Virol. 2019. PMID: 31597767 Free PMC article.
-
Viral Determinants in H5N1 Influenza A Virus Enable Productive Infection of HeLa Cells.J Virol. 2020 Jan 31;94(4):e01410-19. doi: 10.1128/JVI.01410-19. Print 2020 Jan 31. J Virol. 2020. PMID: 31776276 Free PMC article.
-
Human C1q Regulates Influenza A Virus Infection and Inflammatory Response via Its Globular Domain.Int J Mol Sci. 2022 Mar 11;23(6):3045. doi: 10.3390/ijms23063045. Int J Mol Sci. 2022. PMID: 35328462 Free PMC article.
-
Comparison of the pathology caused by H1N1, H5N1, and H3N2 influenza viruses.Arch Med Res. 2009 Nov;40(8):655-61. doi: 10.1016/j.arcmed.2009.10.001. Epub 2010 Jan 6. Arch Med Res. 2009. PMID: 20304252 Review.
Cited by
-
Lung Organoids: Systematic Review of Recent Advancements and its Future Perspectives.Tissue Eng Regen Med. 2024 Jul;21(5):653-671. doi: 10.1007/s13770-024-00628-2. Epub 2024 Mar 11. Tissue Eng Regen Med. 2024. PMID: 38466362 Free PMC article.
-
Porcine Airway Organoid-Derived Well-Differentiated Epithelial Cultures as a Tool for the Characterization of Swine Influenza a Virus Strains.Viruses. 2024 Nov 15;16(11):1777. doi: 10.3390/v16111777. Viruses. 2024. PMID: 39599891 Free PMC article.
-
Berberine Suppresses Influenza A Virus-Triggered Pyroptosis in Macrophages via Intervening in the mtROS-MAVS-NLRP3 Inflammasome Pathway.Viruses. 2025 Apr 7;17(4):539. doi: 10.3390/v17040539. Viruses. 2025. PMID: 40284982 Free PMC article.
-
Examining longitudinal associations between prenatal exposure to infections and child brain morphology.Brain Behav Immun. 2024 Jul;119:965-977. doi: 10.1016/j.bbi.2024.05.014. Epub 2024 May 13. Brain Behav Immun. 2024. PMID: 38750701 Free PMC article.
-
The Role of Inflammation in the Pathogenesis of Viral Respiratory Infections.Microorganisms. 2024 Dec 7;12(12):2526. doi: 10.3390/microorganisms12122526. Microorganisms. 2024. PMID: 39770727 Free PMC article. Review.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
