Irreversible electroporation augments β-glucan induced trained innate immunity for the treatment of pancreatic ductal adenocarcinoma
- PMID: 37072351
- PMCID: PMC10124260
- DOI: 10.1136/jitc-2022-006221
Irreversible electroporation augments β-glucan induced trained innate immunity for the treatment of pancreatic ductal adenocarcinoma
Abstract
Background: Pancreatic cancer (PC) is a challenging diagnosis that is yet to benefit from the advancements in immuno-oncologic treatments. Irreversible electroporation (IRE), a non-thermal method of tumor ablation, is used in treatment of select patients with locally-advanced unresectable PC and has potentiated the effect of certain immunotherapies. Yeast-derived particulate β-glucan induces trained innate immunity and successfully reduces murine PC tumor burden. This study tests the hypothesis that IRE may augment β-glucan induced trained immunity in the treatment of PC.
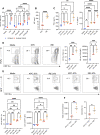
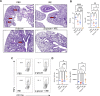
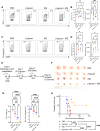
Methods: β-Glucan-trained pancreatic myeloid cells were evaluated ex vivo for trained responses and antitumor function after exposure to ablated and unablated tumor-conditioned media. β-Glucan and IRE combination therapy was tested in an orthotopic murine PC model in wild-type and Rag-/- mice. Tumor immune phenotypes were assessed by flow cytometry. Effect of oral β-glucan in the murine pancreas was evaluated and used in combination with IRE to treat PC. The peripheral blood of patients with PC taking oral β-glucan after IRE was evaluated by mass cytometry.
Results: IRE-ablated tumor cells elicited a potent trained response ex vivo and augmented antitumor functionality. In vivo, β-glucan in combination with IRE reduced local and distant tumor burden prolonging survival in a murine orthotopic PC model. This combination augmented immune cell infiltration to the PC tumor microenvironment and potentiated the trained response from tumor-infiltrating myeloid cells. The antitumor effect of this dual therapy occurred independent of the adaptive immune response. Further, orally administered β-glucan was identified as an alternative route to induce trained immunity in the murine pancreas and prolonged PC survival in combination with IRE. β-Glucan in vitro treatment also induced trained immunity in peripheral blood monocytes obtained from patients with treatment-naïve PC. Finally, orally administered β-glucan was found to significantly alter the innate cell landscape within the peripheral blood of five patients with stage III locally-advanced PC who had undergone IRE.
Conclusions: These data highlight a relevant and novel application of trained immunity within the setting of surgical ablation that may stand to benefit patients with PC.
Keywords: Immunity, Innate; Immunotherapy; Phagocytosis; Tumor Microenvironment.
© Author(s) (or their employer(s)) 2023. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: RCGM is an educational consultant for AngioDynamics.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
