Barriers to immune cell infiltration in tumors
- PMID: 37072352
- PMCID: PMC10124321
- DOI: 10.1136/jitc-2022-006401
Barriers to immune cell infiltration in tumors
Abstract
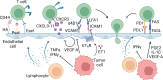
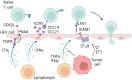
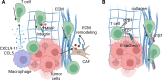
Increased immune cell infiltration into tumors is associated with improved patient survival and predicts response to immune therapies. Thus, identification of factors that determine the extent of immune infiltration is crucial, so that methods to intervene on these targets can be developed. T cells enter tumor tissues through the vasculature, and under control of interactions between homing receptors on the T cells and homing receptor ligands (HRLs) expressed by tumor vascular endothelium and tumor cell nests. HRLs are often deficient in tumors, and there also may be active barriers to infiltration. These remain understudied but may be crucial for enhancing immune-mediated cancer control. Multiple intratumoral and systemic therapeutic approaches show promise to enhance T cell infiltration, including both approved therapies and experimental therapies. This review highlights the intracellular and extracellular determinants of immune cell infiltration into tumors, barriers to infiltration, and approaches for intervention to enhance infiltration and response to immune therapies.
Keywords: CD8-positive T-lymphocytes; cytotoxicity, immunologic; lymphocytes, tumor-infiltrating; tumor microenvironment.
© Author(s) (or their employer(s)) 2023. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: The following disclosures apply to CLS, but are not related to this work: research support to the University of Virginia from Celldex (funding, drug), GlaxoSmithKline (funding), Merck (funding, drug), 3M (drug), Theraclion (device staff support), Polynoma (PI of clinical trial of melanoma vaccine); funding to the University of Virginia for Scientific Advisory Boards for Immatics and CureVac. Also CLS receives license fee payments through the UVA Licensing and Ventures Group for patents for peptides used in cancer vaccines. No potential conflicts of interest were disclosed by MMM, NDS, and KML.
Figures
References
-
- Mihm MCJ, Clemente CG, Cascinelli N. Tumor infiltrating lymphocytes in lymph node melanoma metastases: a histopathologic prognostic indicator and an expression of local immune response. Lab Invest 1996;74:43–7. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
