Sensitivity and specificity of CRP and symptom screening as tuberculosis screening tools among HIV-positive and negative outpatients at a primary healthcare facility in Lusaka, Zambia: a prospective cross-sectional study
- PMID: 37072353
- PMCID: PMC10124229
- DOI: 10.1136/bmjopen-2022-061907
Sensitivity and specificity of CRP and symptom screening as tuberculosis screening tools among HIV-positive and negative outpatients at a primary healthcare facility in Lusaka, Zambia: a prospective cross-sectional study
Abstract
Objectives: To evaluate the performance of point-of-care C-reactive protein (CRP) as a screening tool for tuberculosis (TB) using a threshold of 10 mg/L in both people living with HIV (PLHIV) and HIV-negative individuals and compare it to symptom screening using a composite reference for bacteriological confirmation of TB.
Methods: Prospective cross-sectional study.
Setting: A primary healthcare facility in Lusaka, Zambia.
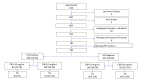
Participants: Consecutive adults (≥18 years) presenting for routine outpatient healthcare were enrolled. Of the 816 individuals approached to participate in the study, 804 eligible consenting adults were enrolled into the study, of which 783 were included in the analysis.
Primary outcome measures: Sensitivity, specificity, positive predictive value and negative predictive value (NPV) of CRP and symptom screening.
Results: Overall, sensitivity of WHO-recommended four-symptom screen (W4SS) and CRP were 87.2% (80.0-92.5) and 86.6% (79.6-91.8) while specificity was 30.3% (26.7-34.1) and 34.8% (31.2-38.6), respectively. Among PLHIV, sensitivity of W4SS and CRP was 92.2% (81.1-97.8) and 94.8% (85.6-98.9) while specificity was 37.0% (31.3-43.0) and 27.5% (22.4-33.1), respectively. Among those with CD4≥350, the NPV for CRP was 100% (92.9-100). In the HIV negative, sensitivity of W4SS and CRP was 83.8% (73.4-91.3) and 80.3% (69.5-88.5) while specificity was 25.4% (20.9-30.2) and 40.5% (35.3-45.6), respectively. Parallel use of CRP and W4SS yielded a sensitivity and NPV of 100% (93.8-100) and 100% (91.6-100) among PLHIV and 93.3% (85.1-97.8) and 90.0% (78.2-96.7) among the HIV negatives, respectively.
Conclusion: Sensitivity and specificity of CRP were similar to symptom screening in HIV-positive outpatients. Independent use of CRP offered limited additional benefit in the HIV negative. CRP can independently accurately rule out TB in PLHIV with CD4≥350. Parallel use of CRP and W4SS improves sensitivity irrespective of HIV status and can accurately rule out TB in PLHIV, irrespective of CD4 count.
Keywords: HIV & AIDS; Public health; Tuberculosis.
© Author(s) (or their employer(s)) 2023. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
Similar articles
-
Tuberculosis screening among ambulatory people living with HIV: a systematic review and individual participant data meta-analysis.Lancet Infect Dis. 2022 Apr;22(4):507-518. doi: 10.1016/S1473-3099(21)00387-X. Epub 2021 Nov 17. Lancet Infect Dis. 2022. PMID: 34800394 Free PMC article.
-
Use of point-of-care C-reactive protein testing for screening of tuberculosis in the community in high-burden settings: a prospective, cross-sectional study in Zambia and South Africa.Lancet Glob Health. 2023 May;11(5):e704-e714. doi: 10.1016/S2214-109X(23)00113-4. Lancet Glob Health. 2023. PMID: 37061309
-
Point-of-care C-reactive protein-based tuberculosis screening for people living with HIV: a diagnostic accuracy study.Lancet Infect Dis. 2017 Dec;17(12):1285-1292. doi: 10.1016/S1473-3099(17)30488-7. Epub 2017 Aug 25. Lancet Infect Dis. 2017. PMID: 28847636 Free PMC article.
-
Impact of hematocrit on point-of-care C-reactive protein-based tuberculosis screening among people living with HIV initiating antiretroviral therapy in Uganda.Diagn Microbiol Infect Dis. 2021 Mar;99(3):115281. doi: 10.1016/j.diagmicrobio.2020.115281. Epub 2020 Nov 26. Diagn Microbiol Infect Dis. 2021. PMID: 33453673 Free PMC article.
-
Screening performance of C-reactive protein for active pulmonary tuberculosis in HIV-positive patients: A systematic review with a meta-analysis.Front Immunol. 2022 Aug 25;13:891201. doi: 10.3389/fimmu.2022.891201. eCollection 2022. Front Immunol. 2022. PMID: 36090970 Free PMC article.
Cited by
-
Evaluation of C-Reactive Protein and Computer-Aided Analysis of Chest X-rays as Tuberculosis Triage Tests at Health Facilities in Lesotho and South Africa.Clin Infect Dis. 2024 Nov 22;79(5):1293-1302. doi: 10.1093/cid/ciae378. Clin Infect Dis. 2024. PMID: 39190813 Free PMC article. Clinical Trial.
-
Comparative Study on the Efficacy of Two Perioperative Chemotherapy Regimens for Lumbar Brucellosis.Drug Des Devel Ther. 2023 Nov 27;17:3523-3536. doi: 10.2147/DDDT.S427477. eCollection 2023. Drug Des Devel Ther. 2023. PMID: 38046280 Free PMC article.
-
Differentiating Lung Adenocarcinoma from Tuberculous Nodules in HIV/AIDS Patients Using Preoperative CT-Based Intratumoral and Peritumoral Radiomics Combined with Clinical Features.J Multidiscip Healthc. 2025 May 12;18:2693-2706. doi: 10.2147/JMDH.S524527. eCollection 2025. J Multidiscip Healthc. 2025. PMID: 40384812 Free PMC article.
-
The accuracy of point-of-care C-Reactive Protein as a screening test for tuberculosis in children.PLOS Glob Public Health. 2024 Oct 24;4(10):e0003725. doi: 10.1371/journal.pgph.0003725. eCollection 2024. PLOS Glob Public Health. 2024. PMID: 39446791 Free PMC article.
-
Clinic presentation delay and tuberculosis treatment outcomes in the Lake Victoria region of East Africa: A multi-site prospective cohort study.PLOS Glob Public Health. 2023 Aug 30;3(8):e0002259. doi: 10.1371/journal.pgph.0002259. eCollection 2023. PLOS Glob Public Health. 2023. PMID: 37647287 Free PMC article.
References
-
- Kwas H, Guermazi E, Zendah I, et al. C-reactive protein and pulmonary tuberculosis: what correlation with disease severity. Annual Congress 2015; Sep, 2015. - DOI
-
- Choi CM, Kang CI, Jeung WK, et al. Role of the C-reactive protein for the diagnosis of TB among military personnel in South Korea. Int J Tuberc Lung Dis. 2007;11:233–6. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous