CBGA ameliorates inflammation and fibrosis in nephropathy
- PMID: 37072467
- PMCID: PMC10113213
- DOI: 10.1038/s41598-023-33507-2
CBGA ameliorates inflammation and fibrosis in nephropathy
Abstract
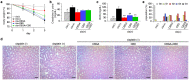
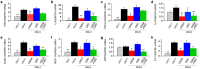
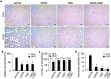
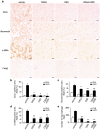
Cannabidiol (CBD) is thought to have multiple biological effects, including the ability to attenuate inflammatory processes. Cannabigerols (CBGA and its decarboxylated CBG molecule) have pharmacological profiles similar to CBD. The endocannabinoid system has recently emerged to contribute to kidney disease, however, the therapeutic properties of cannabinoids in kidney disease remain largely unknown. In this study, we determined whether CBD and CBGA can attenuate kidney damage in an acute kidney disease model induced by the chemotherapeutic cisplatin. In addition, we evaluated the anti-fibrosis effects of these cannabinoids in a chronic kidney disease model induced by unilateral ureteral obstruction (UUO). We find that CBGA, but not CBD, protects the kidney from cisplatin-induced nephrotoxicity. CBGA also strongly suppressed mRNA of inflammatory cytokines in cisplatin-induced nephropathy, whereas CBD treatment was only partially effective. Furthermore, both CBGA and CBD treatment significantly reduced apoptosis through inhibition of caspase-3 activity. In UUO kidneys, both CBGA and CBD strongly reduced renal fibrosis. Finally, we find that CBGA, but not CBD, has a potent inhibitory effect on the channel-kinase TRPM7. We conclude that CBGA and CBD possess reno-protective properties, with CBGA having a higher efficacy, likely due to its dual anti-inflammatory and anti-fibrotic effects paired with TRPM7 inhibition.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

