Tumor resident memory CD8 T cells and concomitant tumor immunity develop independently of CD4 help
- PMID: 37072485
- PMCID: PMC10113239
- DOI: 10.1038/s41598-023-33508-1
Tumor resident memory CD8 T cells and concomitant tumor immunity develop independently of CD4 help
Abstract
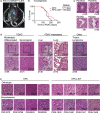
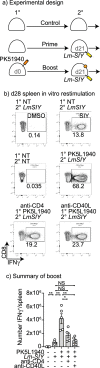
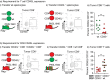
Tissue resident memory (Trm) CD8 T cells infiltrating tumors represent an enriched population of tumor antigen-specific T cells, and their presence is associated with improved outcomes in patients. Using genetically engineered mouse pancreatic tumor models we demonstrate that tumor implantation generates a Trm niche that is dependent on direct antigen presentation by cancer cells. However, we observe that initial CCR7-mediated localization of CD8 T cells to tumor draining lymph nodes is required to subsequently generate CD103+ CD8 T cells in tumors. We observe that the formation of CD103+ CD8 T cells in tumors is dependent on CD40L but independent of CD4 T cells, and using mixed chimeras we show that CD8 T cells can provide their own CD40L to permit CD103+ CD8 T cell differentiation. Finally, we show that CD40L is required to provide systemic protection against secondary tumors. These data suggest that CD103+ CD8 T cell formation in tumors can occur independent of the two-factor authentication provided by CD4 T cells and highlight CD103+ CD8 T cells as a distinct differentiation decision from CD4-dependent central memory.
© 2023. The Author(s).
Conflict of interest statement
MJG receives research funding from Bristol Myers Squibb and VIR Biotechnology. KY receives research funding from Bristol Myers Squibb and consulting fees/stock from Synthis Therapeutics. MRC receives consulting fees from Roche. All other authors have no conflicts to declare. Research funding is not directly related to the topic of this manuscript. Funders had no role in the representation of the data or the preparation of the manuscript.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

