Cocatalyst loaded Al-SrTiO3 cubes for Congo red dye photo-degradation under wide range of light
- PMID: 37072527
- PMCID: PMC10113377
- DOI: 10.1038/s41598-023-33249-1
Cocatalyst loaded Al-SrTiO3 cubes for Congo red dye photo-degradation under wide range of light
Abstract
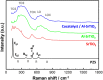
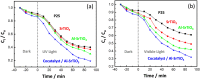
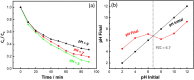
The continued pollution, waste, and unequal distribution of the limited amount of fresh water on earth are pushing the world into water scarcity crisis. Consequently, development of revolutionary, cost-effective, and efficient techniques for water purification is essential. Herein, molten flux method was used for the preparation of micro-sized Al-doped SrTiO3 photocatalyst loaded with RhCr2O3 and CoOOH cocatalysts via simple impregnation method for the photo-assisted degradation of Congo red dye under UV and visible irradiation compared with P25 standard photocatalyst. In addition, photoelectrochemical analysis was conducted to reveal the separation and transfer efficiency of the photogenerated e-/h+ pairs playing the key role in photocatalysis. SEM and TEM analyses revealed that both P25 and the pristine SrTiO3 have spherical shapes, while Al-doped SrTiO3 and the sample loaded with cocatalysts have cubic shapes with a relatively higher particle size reaching 145 nm. In addition, the lowest bandgap is due to Al+3 ion doping and excessive surface oxygen vacancies, as confirmed by both UV-Vis diffuse-reflectance and XPS analyses. The loading of the cocatalysts resulted in a change in the bandgap from n-type (pristine SrTiO3 and Al-SrTiO3) into p-type (cocatalyst loaded sample) as exhibited by Mott-Schottky plots. Besides, the cocatalyst-loaded sample exhibited good performance stability after 5 cycles of the photocatalytic removal of Congo red dye. OH· radical was the primary species responsible for CR degradation as confirmed by experiments with radical scavengers. The observed performance of the prepared samples under both UV and visible light could foster the ongoing efforts towards more efficient photocatalysts for water purification.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures







References
-
- Abdelhamid HN, Mathew AP. Cellulose–metal organic frameworks (CelloMOFs) hybrid materials and their multifaceted applications: A review. Coord. Chem. Rev. 2022;451:214263. doi: 10.1016/j.ccr.2021.214263. - DOI
-
- Niu J, et al. Microwave-based preparation of γ-Fe2O3/SrTiO3 photocatalyst for efficient degradation of organic pollutants in water. Mater. Chem. Phys. 2022;288:126357. doi: 10.1016/j.matchemphys.2022.126357. - DOI
-
- Abdel Maksoud MIA, et al. Engineered magnetic oxides nanoparticles as efficient sorbents for wastewater remediation: A review. Environ. Chem. Lett. 2021;20:519–562. doi: 10.1007/s10311-021-01351-3. - DOI
-
- Merrad S, et al. Study of Congo Red removal from aqueous solution by using the deficient perovskite SrTiO3-δ under solar light. J. Mol. Struct. 2022;1265:133349. doi: 10.1016/j.molstruc.2022.133349. - DOI
LinkOut - more resources
Full Text Sources

