Microbiota-derived tryptophan metabolites indole-3-lactic acid is associated with intestinal ischemia/reperfusion injury via positive regulation of YAP and Nrf2
- PMID: 37072757
- PMCID: PMC10111656
- DOI: 10.1186/s12967-023-04109-3
Microbiota-derived tryptophan metabolites indole-3-lactic acid is associated with intestinal ischemia/reperfusion injury via positive regulation of YAP and Nrf2
Abstract
Background: Lactobacillus has been demonstrated to serve a protective role in intestinal injury. However, the relationship between Lactobacillus murinus (L. murinus)-derived tryptophan metabolites and intestinal ischemia/reperfusion (I/R) injury yet to be investigated. This study aimed to evaluate the role of L. murinus-derived tryptophan metabolites in intestinal I/R injury and the underlying molecular mechanism.
Methods: Liquid chromatograph mass spectrometry analysis was used to measure the fecal content of tryptophan metabolites in mice undergoing intestinal I/R injury and in patients undergoing cardiopulmonary bypass (CPB) surgery. Immunofluorescence, quantitative RT-PCR, Western blot, and ELISA were performed to explore the inflammation protective mechanism of tryptophan metabolites in WT and Nrf2-deficient mice undergoing intestinal I/R, hypoxia-reoxygenation (H/R) induced intestinal organoids.
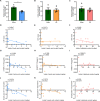
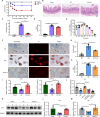
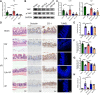
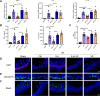
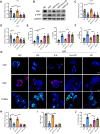
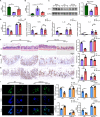
Results: By comparing the fecal contents of three L. murinus-derived tryptophan metabolites in mice undergoing intestinal I/R injury and in patients undergoing cardiopulmonary bypass (CPB) surgery. We found that the high abundance of indole-3-lactic acid (ILA) in the preoperative feces was associated with better postoperative intestinal function, as evidenced by the correlation of fecal metabolites with postoperative gastrointestinal function, serum I-FABP and D-Lactate levels. Furthermore, ILA administration improved epithelial cell damage, accelerated the proliferation of intestinal stem cells, and alleviated the oxidative stress of epithelial cells. Mechanistically, ILA improved the expression of Yes Associated Protein (YAP) and Nuclear Factor erythroid 2-Related Factor 2 (Nrf2) after intestinal I/R. The YAP inhibitor verteporfin (VP) reversed the anti-inflammatory effect of ILA, both in vivo and in vitro. Additionally, we found that ILA failed to protect epithelial cells from oxidative stress in Nrf2 knockout mice under I/R injury.
Conclusions: The content of tryptophan metabolite ILA in the preoperative feces of patients is negatively correlated with intestinal function damage under CPB surgery. Administration of ILA alleviates intestinal I/R injury via the regulation of YAP and Nrf2. This study revealed a novel therapeutic metabolite and promising candidate targets for intestinal I/R injury treatment.
Keywords: Indole-3-lactic acid; Intestinal ischemia/reperfusion injury; Nrf2; Oxidative stress; YAP.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interest in performing this study.
Figures
Similar articles
-
Mechanisms and Therapeutic Potential of Key Anti-inflammatory Metabiotics: Trans-Vaccenic Acid, Indole-3-Lactic Acid, Thiamine, and Butyric Acid.Probiotics Antimicrob Proteins. 2025 Feb 8. doi: 10.1007/s12602-025-10475-9. Online ahead of print. Probiotics Antimicrob Proteins. 2025. PMID: 39921846 Review.
-
Lactobacillus murinus alleviate intestinal ischemia/reperfusion injury through promoting the release of interleukin-10 from M2 macrophages via Toll-like receptor 2 signaling.Microbiome. 2022 Mar 3;10(1):38. doi: 10.1186/s40168-022-01227-w. Microbiome. 2022. PMID: 35241180 Free PMC article.
-
Indole-3-lactic acid associated with Bifidobacterium-dominated microbiota significantly decreases inflammation in intestinal epithelial cells.BMC Microbiol. 2020 Nov 23;20(1):357. doi: 10.1186/s12866-020-02023-y. BMC Microbiol. 2020. PMID: 33225894 Free PMC article.
-
β-Patchoulene Preconditioning Protects Mice Against Hepatic Ischemia-Reperfusion Injury by Regulating Nrf2/HO-1 Signaling Pathway.J Surg Res. 2022 Jul;275:161-171. doi: 10.1016/j.jss.2022.02.001. Epub 2022 Mar 10. J Surg Res. 2022. PMID: 35279582
-
Intestinal microbiota imbalance resulted by anti-Toxoplasma gondii immune responses aggravate gut and brain injury.Parasit Vectors. 2024 Jul 2;17(1):284. doi: 10.1186/s13071-024-06349-8. Parasit Vectors. 2024. PMID: 38956725 Free PMC article.
Cited by
-
Attenuation of intestinal ischemia-reperfusion-injury by anesthetics: a potentially protective effect of anesthetic management in experimental studies.Front Pharmacol. 2024 Feb 20;15:1367170. doi: 10.3389/fphar.2024.1367170. eCollection 2024. Front Pharmacol. 2024. PMID: 38444936 Free PMC article. Review.
-
Comprehensive characterization of multi-omics landscapes between gut microbial metabolites and the druggable genome in sepsis.Front Immunol. 2025 Jul 21;16:1597676. doi: 10.3389/fimmu.2025.1597676. eCollection 2025. Front Immunol. 2025. PMID: 40761792 Free PMC article.
-
Identification and Characterization of Genes Associated with Intestinal Ischemia-Reperfusion Injury and Oxidative Stress: A Bioinformatics and Experimental Approach Integrating High-Throughput Sequencing, Machine Learning, and Validation.J Inflamm Res. 2025 Jan 16;18:701-722. doi: 10.2147/JIR.S500360. eCollection 2025. J Inflamm Res. 2025. PMID: 39835298 Free PMC article.
-
Indole Lactic Acid in Plasma and Urine: A Potential Biomarker for Chronic Kidney Disease and Inflammatory.J Inflamm Res. 2024 Jun 26;17:4105-4116. doi: 10.2147/JIR.S458881. eCollection 2024. J Inflamm Res. 2024. PMID: 38948195 Free PMC article.
-
Mechanisms and Therapeutic Potential of Key Anti-inflammatory Metabiotics: Trans-Vaccenic Acid, Indole-3-Lactic Acid, Thiamine, and Butyric Acid.Probiotics Antimicrob Proteins. 2025 Feb 8. doi: 10.1007/s12602-025-10475-9. Online ahead of print. Probiotics Antimicrob Proteins. 2025. PMID: 39921846 Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

