Analysis of patients with colorectal cancer shows a specific increase in serum anti-ING1 autoantibody levels
- PMID: 37072777
- PMCID: PMC10111810
- DOI: 10.1186/s12885-023-10845-y
Analysis of patients with colorectal cancer shows a specific increase in serum anti-ING1 autoantibody levels
Abstract
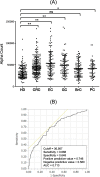
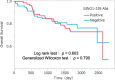
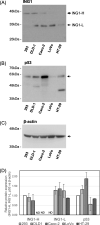
Colorectal cancer (CRC) is the third most prevalent cancer in the world, yet the sensitivity and specificity of biomarkers for CRC diagnosis are insufficient. In the present study, we performed a protein microarray screening method to identify antibody markers for CRC. Inhibitor of growth family 1 (ING1) was identified as a candidate tumor antigen for CRC using protein microarrays (ProtoArray). Subsequent amplified luminescence proximity homogeneous assay-linked immunosorbent assay using recombinant ING1 protein showed that the serum levels of anti-ING1 antibodies were increased not only in patients with CRC but also in those with esophageal cancer (EC), gastric cancer (GC), breast cancer (BrC), and pancreatic cancer (PC) compared with those of healthy donors (HDs). Antibodies against the ING1 amino acids between 239 and 253 were present at significantly higher levels in patients with CRC than in those with EC, GC, BrC, or PC. Anti-ING1 antibody levels were significantly higher in the patients with CRC at any stages than in the HDs. Immunohistochemical staining revealed higher expression of ING1 protein in CRC cells than in the adjacent normal tissues. In luciferase reporter assays using a CRC cell line, ING1 augmented p53-mediated NOXA promoter activity but attenuated p53-stimulated Bax, p21, and PUMA promoter activities. Consequently, serum anti-ING1 antibodies can be used for sensitive and specific diagnoses of CRC.
Keywords: Antibody; Colorectal cancer; Inhibitor of growth protein 1; Protein array analysis; Tumor biomarker.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures






Similar articles
-
The p53 tumor suppressor is stabilized by inhibitor of growth 1 (ING1) by blocking polyubiquitination.PLoS One. 2011;6(6):e21065. doi: 10.1371/journal.pone.0021065. Epub 2011 Jun 22. PLoS One. 2011. PMID: 21731648 Free PMC article.
-
Reduced expression of p33(ING1) and the relationship with p53 expression in human gastric cancer.Cancer Lett. 1999 Dec 1;147(1-2):157-62. doi: 10.1016/s0304-3835(99)00288-8. Cancer Lett. 1999. PMID: 10660101
-
Diminished expression of ING1 mRNA and the correlation with p53 expression in breast cancers.Cancer Lett. 2000 Apr 28;152(1):15-22. doi: 10.1016/s0304-3835(99)00434-6. Cancer Lett. 2000. PMID: 10754201
-
Anti-p53 autoantibody in blood as a diagnostic biomarker for colorectal cancer: A meta-analysis.Scand J Immunol. 2020 Feb;91(2):e12829. doi: 10.1111/sji.12829. Epub 2019 Nov 10. Scand J Immunol. 2020. PMID: 31536647 Review.
-
Different HATS of the ING1 gene family.Trends Cell Biol. 2002 Nov;12(11):532-8. doi: 10.1016/s0962-8924(02)02391-7. Trends Cell Biol. 2002. PMID: 12446115 Review.
Cited by
-
Identification of novel target genes in exaggerated cardiac remodeling following myocardial infarction in diabetes.Front Endocrinol (Lausanne). 2025 Mar 14;16:1536639. doi: 10.3389/fendo.2025.1536639. eCollection 2025. Front Endocrinol (Lausanne). 2025. PMID: 40162308 Free PMC article.
-
Immunohistochemical Analysis of the p53 Protein in Colorectal Cancer: A Clinicopathological Study.Cureus. 2024 Dec 22;16(12):e76172. doi: 10.7759/cureus.76172. eCollection 2024 Dec. Cureus. 2024. PMID: 39840194 Free PMC article.
-
Autoantibody repertoire profiling in tissue and blood identifies colorectal cancer-specific biomarkers.Cancer Sci. 2024 Jan;115(1):83-93. doi: 10.1111/cas.16011. Epub 2023 Nov 20. Cancer Sci. 2024. PMID: 37985391 Free PMC article.
References
-
- Shimada H, Takeda A, Arima M, Okazumi S, Matsubara H, Nabeya Y, Funami Y, Hayashi H, Gunji Y, Suzuki T, et al. Serum p53 antibody is a useful tumor marker in superficial esophageal squamous cell carcinoma. Cancer. 2000;89:1677–1683. doi: 10.1002/1097-0142(20001015)89:8<1677::AID-CNCR5>3.0.CO;2-9. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

