Colorectal cancer-derived small extracellular vesicles induce TGFβ1-mediated epithelial to mesenchymal transition of hepatocytes
- PMID: 37072829
- PMCID: PMC10114452
- DOI: 10.1186/s12935-023-02916-8
Colorectal cancer-derived small extracellular vesicles induce TGFβ1-mediated epithelial to mesenchymal transition of hepatocytes
Abstract
Background: Metastatic disease is the major cause of cancer-related deaths. Increasing evidence shows that primary tumor cells can promote metastasis by preparing the local microenvironment of distant organs, inducing the formation of the so-called "pre-metastatic niche". In recent years, several studies have highlighted that among the tumor-derived molecular components active in pre-metastatic niche formation, small extracellular vesicles (sEVs) play a crucial role. Regarding liver metastasis, the ability of tumor-derived sEVs to affect the activities of non-parenchymal cells such as Kupffer cells and hepatic stellate cells is well described, while the effects on hepatocytes, the most conspicuous and functionally relevant hepatic cellular component, remain unknown.
Methods: sEVs isolated from SW480 and SW620 CRC cells and from clinical samples of CRC patients and healthy subjects were used to treat human healthy hepatocytes (THLE-2 cells). RT-qPCR, Western blot and confocal microscopy were applied to investigate the effects of this treatment.
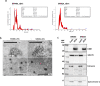
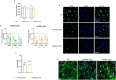
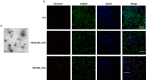
Results: Our study shows for the first time that TGFβ1-carrying CRC_sEVs impair the morphological and functional properties of healthy human hepatocytes by triggering their TGFβ1/SMAD-dependent EMT. These abilities of CRC_sEVs were further confirmed by evaluating the effects elicited on hepatocytes by sEVs isolated from plasma and biopsies from CRC patients.
Conclusions: Since it is known that EMT of hepatocytes leads to the formation of a fibrotic environment, a well-known driver of metastasis, these results suggest that CRC_sEV-educated hepatocytes could have an active and until now neglected role during liver metastasis formation.
Keywords: Colorectal cancer; Hepatocytes; Liver metastasis; Small extracellular vesicles; Transforming growth factor‑β1 (TGFβ1).
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




References
-
- Zhang J, Kumar S, Jayachandran M, Herrera Hernandez LP, Wang S, Wilson EM, et al. Excretion of urine extracellular vesicles bearing markers of activated immune cells and calcium/phosphorus physiology differ between calcium kidney stone formers and non-stone formers. BMC Nephrol. 2021;22(1):204. doi: 10.1186/s12882-021-02417-8. - DOI - PMC - PubMed
-
- Jayachandran M, Yuzhakov SV, Kumar S, Larson NB, Enders FT, Milliner DS, et al. Specific populations of urinary extracellular vesicles and proteins differentiate type 1 primary hyperoxaluria patients without and with nephrocalcinosis or kidney stones. Orphanet J Rare Dis. 2020;15(1):319. doi: 10.1186/s13023-020-01607-1. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

