Antisense oligonucleotides targeting basal forebrain ATXN2 enhances spatial memory and ameliorates sleep deprivation-induced fear memory impairment in mice
- PMID: 37072935
- PMCID: PMC10275523
- DOI: 10.1002/brb3.3013
Antisense oligonucleotides targeting basal forebrain ATXN2 enhances spatial memory and ameliorates sleep deprivation-induced fear memory impairment in mice
Abstract
Introduction: Regulation of brain-derived neurotrophic factor (BDNF) in the basal forebrain ameliorates sleep deprivation-induced fear memory impairments in rodents. Antisense oligonucleotides (ASOs) targeting ATXN2 was a potential therapy for spinocerebellar ataxia, whose pathogenic mechanism associates with reduced BDNF expression. We tested the hypothesis that ASO7 targeting ATXN2 could affect BDNF levels in mouse basal forebrain and ameliorate sleep deprivation-induced fear memory impairments.
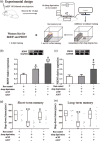
Methods: Adult male C57BL/6 mice were used to evaluate the effects of ASO7 targeting ATXN2 microinjected into the bilateral basal forebrain (1 μg, 0.5 μL, each side) on spatial memory, fear memory and sleep deprivation-induced fear memory impairments. Spatial memory and fear memory were detected by the Morris water maze and step-down inhibitory avoidance test, respectively. Immunohistochemistry, RT-PCR, and Western blot were used to evaluate the changes of levels of BDNF, ATXN2, and postsynaptic density 95 (PSD95) protein as well as ATXN2 mRNA. The morphological changes in neurons in the hippocampal CA1 region were detected by HE staining and Nissl staining.
Results: ASO7 targeting ATXN2 microinjected into the basal forebrain could suppress ATXN2 mRNA and protein expression for more than 1 month and enhance spatial memory but not fear memory in mice. BDNF mRNA and protein expression in basal forebrain and hippocampus was increased by ASO7. Moreover, PSD95 expression and synapse formation were increased in the hippocampus. Furthermore, ASO7 microinjected into the basal forebrain increased BDNF and PSD95 protein expression in the basal forebrain of sleep-deprived mice and counteracted sleep deprivation-induced fear memory impairments.
Conclusion: ASOs targeting ATXN2 may provide effective interventions for sleep deprivation-induced cognitive impairments.
Keywords: ATXN2; antisense oligonucleotides; memory; sleep deprivation.
© 2023 The Authors. Brain and Behavior published by Wiley Periodicals LLC.
Conflict of interest statement
The authors have no conflict of interest to declare.
Figures


Similar articles
-
Activation of brain-derived neurotrophic factor signaling in the basal forebrain reverses acute sleep deprivation-induced fear memory impairments.Brain Behav. 2020 Apr;10(4):e01592. doi: 10.1002/brb3.1592. Epub 2020 Mar 10. Brain Behav. 2020. PMID: 32157827 Free PMC article.
-
Enriched environment ameliorates fear memory impairments induced by sleep deprivation via inhibiting PIEZO1/calpain/autophagy signaling pathway in the basal forebrain.CNS Neurosci Ther. 2024 Feb;30(2):e14365. doi: 10.1111/cns.14365. Epub 2023 Jul 23. CNS Neurosci Ther. 2024. PMID: 37485782 Free PMC article.
-
Inhibition of Piezo1/Ca2+/calpain signaling in the rat basal forebrain reverses sleep deprivation-induced fear memory impairments.Behav Brain Res. 2022 Jan 24;417:113594. doi: 10.1016/j.bbr.2021.113594. Epub 2021 Sep 22. Behav Brain Res. 2022. PMID: 34560129
-
The administration of rhBmal1 reduces sleep deprivation-induced anxiety and cognitive impairment in mice.World J Biol Psychiatry. 2024 Jan-Feb;25(1):43-53. doi: 10.1080/15622975.2023.2252499. Epub 2023 Aug 28. World J Biol Psychiatry. 2024. PMID: 37640026 Review.
-
Recent Insights into Hippocampal Dysfunction and Neuroplasticity in Sleep Disorders: An Update from Preclinical Studies.J Integr Neurosci. 2024 Aug 13;23(8):144. doi: 10.31083/j.jin2308144. J Integr Neurosci. 2024. PMID: 39207067 Review.
Cited by
-
Systemic Neurodegeneration and Brain Aging: Multi-Omics Disintegration, Proteostatic Collapse, and Network Failure Across the CNS.Biomedicines. 2025 Aug 20;13(8):2025. doi: 10.3390/biomedicines13082025. Biomedicines. 2025. PMID: 40868276 Free PMC article. Review.
References
-
- Bawari, S. , Tewari, D. , Argüelles, S. , Sah, A. N. , Nabavi, S. F. , Xu, S. , & Shirooie, S. (2019). Targeting BDNF signaling by natural products: Novel synaptic repair therapeutics for neurodegeneration and behavior disorders. Pharmacological Research, 148, 104458. 10.1016/j.phrs.2019.104458 - DOI - PubMed
-
- Campos‐Jurado, Y. , Igual‐Lopez, M. , Padilla, F. , Zornoza, T. , Granero, L. , Polache, A. , & Hipolito, L. (2019). Activation of MORs in the VTA induces changes on cFos expression in different projecting regions: Effect of inflammatory pain. Neurochemistry International, 131, 104521. 10.1016/j.neuint.2019.104521 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

