CSF2 upregulates CXCL3 expression in adipocytes to promote metastasis of breast cancer via the FAK signaling pathway
- PMID: 37073091
- PMCID: PMC10686244
- DOI: 10.1093/jmcb/mjad025
CSF2 upregulates CXCL3 expression in adipocytes to promote metastasis of breast cancer via the FAK signaling pathway
Abstract
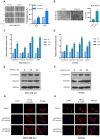
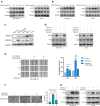
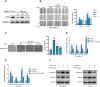
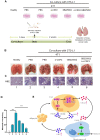
Recent studies have demonstrated that cancer-associated adipocytes (CAAs) in the tumor microenvironment are involved in the malignant progression of breast cancer. However, the underlying mechanism of CAA formation and its effects on the development of breast cancer are still unknown. Here, we show that CSF2 is highly expressed in both CAAs and breast cancer cells. CSF2 promotes inflammatory phenotypic changes of adipocytes through the Stat3 signaling pathway, leading to the secretion of multiple cytokines and proteases, particularly C-X-C motif chemokine ligand 3 (CXCL3). Adipocyte-derived CXCL3 binds to its specific receptor CXCR2 on breast cancer cells and activates the FAK pathway, enhancing the mesenchymal phenotype, migration, and invasion of breast cancer cells. In addition, a combination treatment targeting CSF2 and CXCR2 shows a synergistic inhibitory effect on adipocyte-induced lung metastasis of mouse 4T1 cells in vivo. These findings elucidate a novel mechanism of breast cancer metastasis and provide a potential therapeutic strategy for breast cancer metastasis.
Keywords: CSF2; CXCL3; FAK; breast cancer metastasis; cancer-associated adipocytes.
© The Author(s) (2023). Published by Oxford University Press on behalf of Journal of Molecular Cell Biology, CEMCS, CAS.
Figures




Similar articles
-
Cancer-associated adipocytes promote the invasion and metastasis in breast cancer through LIF/CXCLs positive feedback loop.Int J Biol Sci. 2022 Jan 16;18(4):1363-1380. doi: 10.7150/ijbs.65227. eCollection 2022. Int J Biol Sci. 2022. PMID: 35280694 Free PMC article.
-
Adipocyte-derived IL-6 and leptin promote breast Cancer metastasis via upregulation of Lysyl Hydroxylase-2 expression.Cell Commun Signal. 2018 Dec 18;16(1):100. doi: 10.1186/s12964-018-0309-z. Cell Commun Signal. 2018. PMID: 30563531 Free PMC article.
-
Tumor-secreted PAI-1 promotes breast cancer metastasis via the induction of adipocyte-derived collagen remodeling.Cell Commun Signal. 2019 Jun 6;17(1):58. doi: 10.1186/s12964-019-0373-z. Cell Commun Signal. 2019. PMID: 31170987 Free PMC article.
-
Cancer-Associated Adipocytes in Breast Cancer: Causes and Consequences.Int J Mol Sci. 2021 Apr 6;22(7):3775. doi: 10.3390/ijms22073775. Int J Mol Sci. 2021. PMID: 33917351 Free PMC article. Review.
-
Cancer-associated adipocytes: emerging supporters in breast cancer.J Exp Clin Cancer Res. 2020 Aug 12;39(1):156. doi: 10.1186/s13046-020-01666-z. J Exp Clin Cancer Res. 2020. PMID: 32787888 Free PMC article. Review.
Cited by
-
The evolving tumor-associated adipose tissue microenvironment in breast cancer: from cancer initiation to metastatic outgrowth.Clin Transl Oncol. 2025 Jul;27(7):2778-2788. doi: 10.1007/s12094-024-03831-8. Epub 2024 Dec 25. Clin Transl Oncol. 2025. PMID: 39720985 Review.
-
Coagulation-related genes for thyroid cancer prognosis, immune infltration, staging, and drug sensitivity.Front Immunol. 2024 Oct 21;15:1462755. doi: 10.3389/fimmu.2024.1462755. eCollection 2024. Front Immunol. 2024. PMID: 39497824 Free PMC article.
-
Adipsin-dependent adipocyte maturation induces cancer cell invasion in breast cancer.Sci Rep. 2024 Aug 9;14(1):18494. doi: 10.1038/s41598-024-69476-3. Sci Rep. 2024. PMID: 39122742 Free PMC article.
-
Comprehensive analysis of the clinical and biological significances for chemokine CXCL3 in cholangiocarcinoma.Medicine (Baltimore). 2024 Mar 15;103(11):e37460. doi: 10.1097/MD.0000000000037460. Medicine (Baltimore). 2024. PMID: 38489741 Free PMC article.
-
CXCL3/TGF-β-mediated crosstalk between CAFs and tumor cells augments RCC progression and sunitinib resistance.iScience. 2024 Jun 8;27(7):110224. doi: 10.1016/j.isci.2024.110224. eCollection 2024 Jul 19. iScience. 2024. PMID: 39040058 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

