MicroRNA-216a is essential for cardiac angiogenesis
- PMID: 37073128
- PMCID: PMC10277893
- DOI: 10.1016/j.ymthe.2023.04.007
MicroRNA-216a is essential for cardiac angiogenesis
Abstract
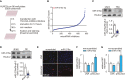
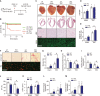
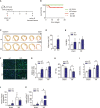
While it is experimentally supported that impaired myocardial vascularization contributes to a mismatch between myocardial oxygen demand and supply, a mechanistic basis for disruption of coordinated tissue growth and angiogenesis in heart failure remains poorly understood. Silencing strategies that impair microRNA biogenesis have firmly implicated microRNAs in the regulation of angiogenesis, and individual microRNAs prove to be crucial in developmental or tumor angiogenesis. A high-throughput functional screening for the analysis of a whole-genome microRNA silencing library with regard to their phenotypic effect on endothelial cell proliferation as a key parameter, revealed several anti- and pro-proliferative microRNAs. Among those was miR-216a, a pro-angiogenic microRNA which is enriched in cardiac microvascular endothelial cells and reduced in expression under cardiac stress conditions. miR-216a null mice display dramatic cardiac phenotypes related to impaired myocardial vascularization and unbalanced autophagy and inflammation, supporting a model where microRNA regulation of microvascularization impacts the cardiac response to stress.
Keywords: angiogenesis; autophagy; cardiac remodeling; endothelial cells; heart failure; microRNAs.
Copyright © 2023 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests L.d.W. and P.d.C.M. are cofounders of Mirabilis Therapeutics.
Figures




References
-
- Diaz-Sandoval L.J., Losordo D.W. Gene therapy for cardiovascular angiogenesis. Expert Opin. Biol. Ther. 2003;3:599–616. - PubMed
-
- Itescu S., Kocher A.A., Schuster M.D. Myocardial neovascularization by adult bone marrow-derived angioblasts: strategies for improvement of cardiomyocyte function. Heart Fail. Rev. 2003;8:253–258. - PubMed
-
- Van der Laan A.M., Piek J.J., Van Royen N. Targeting angiogenesis to restore the microcirculation after reperfused MI. Nat. Rev. Cardiol. 2009;6:515–523. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

