Metabolic strategy of macrophages under homeostasis or immune stress in Drosophila
- PMID: 37073169
- PMCID: PMC10077226
- DOI: 10.1007/s42995-022-00134-1
Metabolic strategy of macrophages under homeostasis or immune stress in Drosophila
Abstract
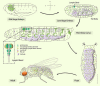
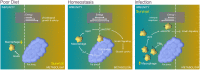
Macrophages are well known for their phagocytic functions in innate immunity across species. In mammals, they rapidly consume a large amount of energy by shifting their metabolism from mitochondrial oxidative phosphorylation toward aerobic glycolysis, to perform the effective bactericidal function upon infection. Meanwhile, they strive for sufficient energy resources by restricting systemic metabolism. In contrast, under nutrient deprivation, the macrophage population is down-regulated to save energy for survival. Drosophila melanogaster possesses a highly conserved and comparatively simple innate immune system. Intriguingly, recent studies have shown that Drosophila plasmatocytes, the macrophage-like blood cells, adopt comparable metabolic remodeling and signaling pathways to achieve energy reassignment when challenged by pathogens, indicating the conservation of such metabolic strategies between insects and mammals. Here, focusing on Drosophila macrophages (plasmatocytes), we review recent advances regarding their comprehensive roles in local or systemic metabolism under homeostasis or stress, emphasizing macrophages as critical players in the crosstalk between the immune system and organic metabolism from a Drosophila perspective.
Keywords: Drosophila; Immune system; Macrophage; Metabolism; Plasmatocyte.
© The Author(s) 2022.
Conflict of interest statement
Conflict of interestThe authors declare no conflict of interest.
Figures


Similar articles
-
Macrophage subpopulation identity in Drosophila is modulated by apoptotic cell clearance and related signalling pathways.Front Immunol. 2024 Jan 12;14:1310117. doi: 10.3389/fimmu.2023.1310117. eCollection 2023. Front Immunol. 2024. PMID: 38283366 Free PMC article.
-
DNA damage signaling in Drosophila macrophages modulates systemic cytokine levels in response to oxidative stress.Elife. 2024 Jan 8;12:RP86700. doi: 10.7554/eLife.86700. Elife. 2024. PMID: 38189792 Free PMC article.
-
Macrophages and cellular immunity in Drosophila melanogaster.Semin Immunol. 2015 Dec;27(6):357-68. doi: 10.1016/j.smim.2016.03.010. Epub 2016 Apr 23. Semin Immunol. 2015. PMID: 27117654 Free PMC article. Review.
-
Macrophages and Their Organ Locations Shape Each Other in Development and Homeostasis - A Drosophila Perspective.Front Cell Dev Biol. 2021 Mar 11;9:630272. doi: 10.3389/fcell.2021.630272. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 33777939 Free PMC article. Review.
-
Drosophila macrophages switch to aerobic glycolysis to mount effective antibacterial defense.Elife. 2019 Oct 14;8:e50414. doi: 10.7554/eLife.50414. Elife. 2019. PMID: 31609200 Free PMC article.
Cited by
-
Dual role of PpV in Drosophila crystal cell proliferation and survival.J Mol Cell Biol. 2025 Mar 21;16(9):mjae028. doi: 10.1093/jmcb/mjae028. J Mol Cell Biol. 2025. PMID: 39085037 Free PMC article.
-
An organ-wide spatiotemporal transcriptomic and cellular atlas of the regenerating zebrafish heart.Nat Commun. 2025 Apr 19;16(1):3716. doi: 10.1038/s41467-025-59070-0. Nat Commun. 2025. PMID: 40253397 Free PMC article.
-
High-throughput screening of caterpillars as a platform to study host-microbe interactions and enteric immunity.Nat Commun. 2022 Nov 24;13(1):7216. doi: 10.1038/s41467-022-34865-7. Nat Commun. 2022. PMID: 36433960 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

