Solutions: how adaptive changes in cellular fluids enable marine life to cope with abiotic stressors
- PMID: 37073170
- PMCID: PMC10077225
- DOI: 10.1007/s42995-022-00140-3
Solutions: how adaptive changes in cellular fluids enable marine life to cope with abiotic stressors
Abstract
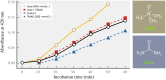
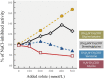
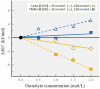
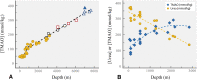
The seas confront organisms with a suite of abiotic stressors that pose challenges for physiological activity. Variations in temperature, hydrostatic pressure, and salinity have potential to disrupt structures, and functions of all molecular systems on which life depends. During evolution, sequences of nucleic acids and proteins are adaptively modified to "fit" these macromolecules for function under the particular abiotic conditions of the habitat. Complementing these macromolecular adaptations are alterations in compositions of solutions that bathe macromolecules and affect stabilities of their higher order structures. A primary result of these "micromolecular" adaptations is preservation of optimal balances between conformational rigidity and flexibility of macromolecules. Micromolecular adaptations involve several families of organic osmolytes, with varying effects on macromolecular stability. A given type of osmolyte generally has similar effects on DNA, RNA, proteins and membranes; thus, adaptive regulation of cellular osmolyte pools has a global effect on macromolecules. These effects are mediated largely through influences of osmolytes and macromolecules on water structure and activity. Acclimatory micromolecular responses are often critical in enabling organisms to cope with environmental changes during their lifetimes, for example, during vertical migration in the water column. A species' breadth of environmental tolerance may depend on how effectively it can vary the osmolyte composition of its cellular fluids in the face of stress. Micromolecular adaptations remain an under-appreciated aspect of evolution and acclimatization. Further study can lead to a better understanding of determinants of environmental tolerance ranges and to biotechnological advances in designing improved stabilizers for biological materials.
Keywords: Adaptation; Crowding; Extremophiles; Hydrostatic pressure; Osmolytes; Temperature.
© The Author(s) 2022.
Conflict of interest statement
Conflict of interestThe author has no competing interests, financial or otherwise.
Figures

References
-
- Anjum R, Nishimura S-N, Kobayashi S, Nishida K, Anada T, Tanaka M. Protein stabilization effect of zwitterionic osmolyte-bearing polymer. Chem Lett. 2021;50:1699–1702. doi: 10.1246/cl.210335. - DOI
Publication types
LinkOut - more resources
Full Text Sources

