Hepatic cecum: a key integrator of immunity in amphioxus
- PMID: 37073295
- PMCID: PMC10077268
- DOI: 10.1007/s42995-020-00080-w
Hepatic cecum: a key integrator of immunity in amphioxus
Abstract
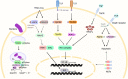
The vertebrate liver is regarded as an organ essential to the regulation of immunity and inflammation as well as being central to the metabolism of nutrients. Here, we discuss the functions that the hepatic cecum of amphioxus plays in the regulation of immunity and inflammation, and the molecular basis of this. It is apparent that the hepatic cecum performs important roles in the immunity of amphioxus including immune surveillance, clearance of pathogens and acute phase response. Therefore, the hepatic cecum, like the vertebrate liver, is an organ functioning as a key integrator of immunity in amphioxus.
Keywords: Acute phase response; Amphioxus; Hepatic cecum; Immunity; Inflammation.
© Ocean University of China 2021.
Conflict of interest statement
Conflict of interestThe authors declare no conflicts of interests.
Figures


Similar articles
-
Identification and expression of liver-specific genes after LPS challenge in amphioxus: the hepatic cecum as liver-like organ and "pre-hepatic" acute phase response.Funct Integr Genomics. 2011 Mar;11(1):111-8. doi: 10.1007/s10142-010-0199-7. Epub 2010 Nov 4. Funct Integr Genomics. 2011. PMID: 21052758
-
Molecular and immunochemical demonstration of a novel member of Bf/C2 homolog in amphioxus Branchiostoma belcheri: implications for involvement of hepatic cecum in acute phase response.Fish Shellfish Immunol. 2008 Jun;24(6):768-78. doi: 10.1016/j.fsi.2008.03.004. Epub 2008 Mar 15. Fish Shellfish Immunol. 2008. PMID: 18434196
-
[Hepatic caecum of amphioxus and origin of vertebrate liver].Yi Chuan. 2010 May;32(5):437-42. doi: 10.3724/sp.j.1005.2010.00437. Yi Chuan. 2010. PMID: 20466630 Review. Chinese.
-
Identification and characterization of a p38-like gene from amphioxus (Branchiostoma belcheri): an insight into amphioxus innate immunity and evolution.Fish Shellfish Immunol. 2014 Dec;41(2):421-7. doi: 10.1016/j.fsi.2014.09.028. Epub 2014 Sep 30. Fish Shellfish Immunol. 2014. PMID: 25281579
-
The amphioxus genome provides unique insight into the evolution of immunity.Brief Funct Genomics. 2012 Mar;11(2):167-76. doi: 10.1093/bfgp/els007. Epub 2012 Mar 7. Brief Funct Genomics. 2012. PMID: 22402506 Free PMC article. Review.
Cited by
-
Contrasting patterns of bacterial communities in the rearing water and gut of Penaeus vannamei in response to exogenous glucose addition.Mar Life Sci Technol. 2022 Jan 1;4(2):222-236. doi: 10.1007/s42995-021-00124-9. eCollection 2022 May. Mar Life Sci Technol. 2022. PMID: 37073217 Free PMC article.
-
Single-cell analysis of the amphioxus hepatic caecum and vertebrate liver reveals genetic mechanisms of vertebrate liver evolution.Nat Ecol Evol. 2024 Oct;8(10):1972-1990. doi: 10.1038/s41559-024-02510-9. Epub 2024 Aug 16. Nat Ecol Evol. 2024. PMID: 39152328
-
Phased chromosome-level genome provides insights into the molecular adaptation for migratory lifestyle and population diversity for Pacific saury, Cololabis saira.Commun Biol. 2024 Nov 15;7(1):1513. doi: 10.1038/s42003-024-07126-0. Commun Biol. 2024. PMID: 39543266 Free PMC article.
-
Liver immunology: Biological role and clinical significance.World J Hepatol. 2025 Jul 27;17(7):107541. doi: 10.4254/wjh.v17.i7.107541. World J Hepatol. 2025. PMID: 40747238 Free PMC article. Review.
References
Publication types
LinkOut - more resources
Full Text Sources

