An insight into the mechanisms of action of selected bioactive compounds against epigenetic targets of prostate cancer: implications on histones modifications
- PMID: 37073308
- PMCID: PMC10105819
- DOI: 10.1007/s40203-023-00148-2
An insight into the mechanisms of action of selected bioactive compounds against epigenetic targets of prostate cancer: implications on histones modifications
Abstract
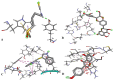
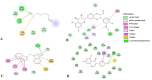
Prostate cancer is a leading cause of morbidity and mortality among men globally. In this study, we employed an in silico approach to predict the possible mechanisms of action of selected novel compounds reported against prostate cancer epigenetic targets and their derivatives, exhausting through ADMET profiling, drug-likeness, and molecular docking analyses. The selected compounds: sulforaphane, silibinin, 3, 3'-diindolylmethane (DIM), and genistein largely conformed to ADMET and drug-likeness rules including Lipinski's. Docking studies revealed strong binding energy of sulforaphane with HDAC6 (- 4.2 kcal/ mol), DIM versus HDAC2 (- 5.2 kcal/mol), genistein versus HDAC6 (- 4.1 kcal/mol), and silibinin against HDAC1 (- 7.0 kcal/mol) coupled with improved binding affinities and biochemical stabilities after derivatization. Findings from this study may provide insight into the potential epigenetic reprogramming mechanisms of these compounds against prostate cancer and could pave the way toward more success in prostate cancer phytotherapy.
Keywords: 3, 3’-diindolylmethane; Bioactive compounds; Epigenetics; Genistein; Prostate cancer; Silibinin; Sulforaphane.
© The Author(s), under exclusive licence to Springer-Verlag GmbH Germany, part of Springer Nature 2023, Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Conflict of interest statement
Competing interestsThe authors declare no competing interests.
Figures
Similar articles
-
In silico predictions on the possible mechanism of action of selected bioactive compounds against breast cancer.In Silico Pharmacol. 2020 Nov 9;8(1):4. doi: 10.1007/s40203-020-00057-8. eCollection 2020. In Silico Pharmacol. 2020. PMID: 33194532 Free PMC article.
-
In silico design of novel bioactive molecules to treat breast cancer with chlorogenic acid derivatives: a computational and SAR approach.Front Pharmacol. 2023 Dec 12;14:1266833. doi: 10.3389/fphar.2023.1266833. eCollection 2023. Front Pharmacol. 2023. PMID: 38152692 Free PMC article.
-
Investigation of the New Inhibitors by Sulfadiazine and Modified Derivatives of α-D-glucopyranoside for White Spot Syndrome Virus Disease of Shrimp by In Silico: Quantum Calculations, Molecular Docking, ADMET and Molecular Dynamics Study.Molecules. 2022 Jun 8;27(12):3694. doi: 10.3390/molecules27123694. Molecules. 2022. PMID: 35744817 Free PMC article.
-
Identification of Kaempferol as Viral Entry Inhibitor and DL-Arginine as Viral Replication Inhibitor from Selected Plants of Indian Traditional Medicine against COVID-19: An in silico Guided in vitro Approach.Curr Comput Aided Drug Des. 2023;19(4):313-323. doi: 10.2174/1573409919666230112123213. Curr Comput Aided Drug Des. 2023. PMID: 36635906
-
Impact of Epigenetic Dietary Components on Cancer through Histone Modifications.Curr Med Chem. 2015;22(17):2051-64. doi: 10.2174/0929867322666150420102641. Curr Med Chem. 2015. PMID: 25891109 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Miscellaneous

