L-Ascorbic acid metabolism and regulation in fruit crops
- PMID: 37073491
- PMCID: PMC10315321
- DOI: 10.1093/plphys/kiad241
L-Ascorbic acid metabolism and regulation in fruit crops
Abstract
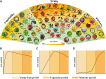
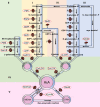
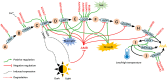
L-Ascorbic acid (AsA) is more commonly known as vitamin C and is an indispensable compound for human health. As a major antioxidant, AsA not only maintains redox balance and resists biological and abiotic stress but also regulates plant growth, induces flowering, and delays senescence through complex signal transduction networks. However, AsA content varies greatly in horticultural crops, especially in fruit crops. The AsA content of the highest species is approximately 1,800 times higher than that of the lowest species. There have been significant advancements in the understanding of AsA accumulation in the past 20 years. The most noteworthy accomplishment was the identification of the critical rate-limiting genes for the 2 major AsA synthesis pathways (L-galactose pathway and D-galacturonic acid pathway) in fruit crops. The rate-limiting genes of the former are GMP, GME, GGP, and GPP, and the rate-limiting gene of the latter is GalUR. Moreover, APX, MDHAR, and DHAR are also regarded as key genes in degradation and regeneration pathways. Interestingly, some of these key genes are sensitive to environmental factors, such as GGP being induced by light. The efficiency of enhancing AsA content is high by editing upstream open reading frames (uORF) of the key genes and constructing multi-gene expression vectors. In summary, the AsA metabolism has been well understood in fruit crops, but the transport mechanism of AsA and the synergistic improvement of AsA and other traits is less known, which will be the focus of AsA research in fruit crops.
© American Society of Plant Biologists 2023. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Conflict of interest statement
Conflict of interest statement. None declared.
Figures

References
-
- Alhagdow M, Mounet F, Gilbert L, Nunes-Nesi A, Garcia V, Just D, Petit J, Beauvoit B, Fernie AR, Rothan C, et al. . Silencing of the mitochondrial ascorbate synthesizing enzyme L-galactono-1,4-lactone dehydrogenase affects plant and fruit development in tomato. Plant Physiol. 2007:145(4):1408–1422. 10.1104/pp.107.106500 - DOI - PMC - PubMed
-
- An HM. Physiological mechanism of accumulating high level L-ascorbic acid, and molecular cloning and expression of its key biosynthetic enzyme in Rosa roxburghii tratt. PhD dissertation. Hangzhou, Zhejiang, China: Zhejiang University; 2004.
-
- An HM, Chen LG, Fan WG. Cloning of cDNA fragment of L-galactono-1,4-lactone dehydrogenase and its expression in different organs of Rosa roxburghii tratt. Scientia Agricultura Sinica. 2005a:38(3):571–575.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

