M2-like macrophages polarized by Foxp3- Treg-of-B cells ameliorate imiquimod-induced psoriasis
- PMID: 37073709
- PMCID: PMC10243160
- DOI: 10.1111/jcmm.17748
M2-like macrophages polarized by Foxp3- Treg-of-B cells ameliorate imiquimod-induced psoriasis
Abstract
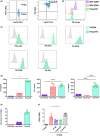
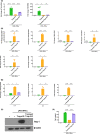
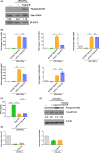
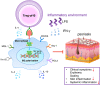
Our group have demonstrated that splenic B cells contributed to the CD4+ CD25- naive T cells conversion into CD4+ CD25+ Foxp3- regulatory T cells without adding appended cytokines, named Treg-of-B cells which were potent suppressors of adaptive immunity. We like to investigate whether Treg-of-B cells could promote alternatively activated macrophage (M2 macrophages) polarization and alleviate inflammatory disease, psoriasis. In this study, we co-cultured the bone marrow-derived macrophages (BMDMs) with Treg-of-B cells under LPS/IFN-γ stimulation and analyzed the M2-associated gene and protein using qPCR, western blotting, and immunofluorescence staining. We also examined the therapeutic effect of Treg-of-B cell-induced M2 macrophage for skin inflammation using imiquimod (IMQ)-induced psoriatic mouse model. Our results showed that BMDMs co-cultured with Treg-of-B cells upregulated typical M2-associated molecules, including Arg-1, IL-10, Pdcd1lg2, MGL-1, IL-4, YM1/2 and CD206. In an inflammatory environment, TNF-α and IL-6 production by macrophages co-cultured with Treg-of-B cells was decreased significantly. The molecular mechanism revealed that Treg-of-B cells promoted M2 macrophage polarization via STAT6 activation in a cell contact-dependent manner. Moreover, the treatment with Treg-of-B cell-induced M2 macrophages attenuated the clinical manifestations of psoriasis, such as scaling, erythema and thickening in the IMQ-induced psoriatic mouse model. T cell activation in draining lymph nodes was decreased in the Treg-of-B cell-induced M2 macrophage group after IMQ application. In conclusion, our findings suggested that Foxp3- Treg-of-B cells could induce alternatively activated M2 macrophages through STAT6 activation, providing a cell-based therapeutic strategy for psoriasis.
Keywords: M2 macrophage; STAT6; Treg-of-B cells; imiquimod; psoriasis.
© 2023 The Authors. Journal of Cellular and Molecular Medicine published by Foundation for Cellular and Molecular Medicine and John Wiley & Sons Ltd.
Conflict of interest statement
The authors have declared no competing interests exist.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

