Convection and extracellular matrix binding control interstitial transport of extracellular vesicles
- PMID: 37073802
- PMCID: PMC10114097
- DOI: 10.1002/jev2.12323
Convection and extracellular matrix binding control interstitial transport of extracellular vesicles
Abstract
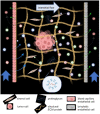
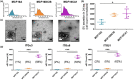
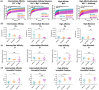
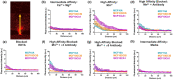
Extracellular vesicles (EVs) influence a host of normal and pathophysiological processes in vivo. Compared to soluble mediators, EVs can traffic a wide range of proteins on their surface including extracellular matrix (ECM) binding proteins, and their large size (∼30-150 nm) limits diffusion. We isolated EVs from the MCF10 series-a model human cell line of breast cancer progression-and demonstrated increasing presence of laminin-binding integrins α3β1 and α6β1 on the EVs as the malignant potential of the MCF10 cells increased. Transport of the EVs within a microfluidic device under controlled physiological interstitial flow (0.15-0.75 μm/s) demonstrated that convection was the dominant mechanism of transport. Binding of the EVs to the ECM enhanced the spatial concentration and gradient, which was mitigated by blocking integrins α3β1 and α6β1. Our studies demonstrate that convection and ECM binding are the dominant mechanisms controlling EV interstitial transport and should be leveraged in nanotherapeutic design.
Keywords: diffusion; exosome; gradient; integrin binding; spatial concentration.
© 2023 The Authors. Journal of Extracellular Vesicles published by Wiley Periodicals, LLC on behalf of the International Society for Extracellular Vesicles.
Conflict of interest statement
There are no conflicts of interest to declare.
Figures


References
-
- Behbod, F. , Kittrell, F. S. , Lamarca, H. , Edwards, D. , Kerbawy, S. , Heestand, J. C. , Young, E. , Mukhopadhyay, P. , Yeh, H.‐W. , Allred, D. C. , Hu, M. , Polyak, K. , Rosen, J. M. , & Medina, D. (2009). An intraductal human‐in‐mouse transplantation model mimics the subtypes of ductal carcinoma in situ. Breast Cancer Res 11, R66. 10.1186/bcr2358 - DOI - PMC - PubMed

