Time-to-event clinical trial designs: Existing evidence and remaining concerns
- PMID: 37073881
- PMCID: PMC10524279
- DOI: 10.1111/epi.17621
Time-to-event clinical trial designs: Existing evidence and remaining concerns
Abstract
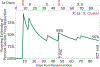
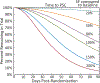
Well-designed placebo-controlled clinical trials are critical to the development of novel treatments for epilepsy, but their design has not changed for decades. Patients, clinicians, regulators, and innovators all have concerns that recruiting for trials is challenging, in part, due to the static design of maintaining participants for long periods on add-on placebo when there are an increasing number of options for therapy. A traditional trial maintains participants on blinded treatment for a static period (e.g., 12 weeks of maintenance), during which participants on placebo have an elevated risk of sudden unexpected death in epilepsy compared to patients on an active treatment. Time-to-event trials observe participants on blinded treatment until a key event occurs (e.g., post-randomization seizure count matches pre-randomization monthly seizure count). In this article, we review the evidence for these designs based on re-analysis of prior trials, one published trial that used a time-to-second seizure design, and experience from an ongoing blinded trial. We also discuss remaining concerns regarding time-to-event trials. We conclude that, despite potential limitations, time-to-event trials are a potential promising mechanism to make trials more patient friendly and reduce placebo exposure, which are urgent needs to improve safety and increase recruitment to trials.
Keywords: pre-randomization seizure count; recruitment; research roundtable for epilepsy (RRE); seizure cycling; sudden unexpected death in epilepsy (SUDEP).
© 2023 The Authors. Epilepsia published by Wiley Periodicals LLC on behalf of International League Against Epilepsy.
Conflict of interest statement
Conflict of Interest Disclosures & Ethical Publication:
Dr. Kerr writes review articles for Medlink Neurology and has consulting agreements with SK Life Science, Janssen, Biohaven Pharmaceutical, and Radius Health. Dr. French receives salary support from the Epilepsy Foundation and for consulting work and/or attending Scientific Advisory Boards on behalf of the Epilepsy Study Consortium for Aeonian/Aeovian, Alterity Therapeutics Limited, Anavex, Arkin Holdings, Angelini Pharma S.p.A, Arvelle Therapeutics, Inc., Athenen Therapeutics/Carnot Pharma, Autifony Therapeutics Limited, Baergic Bio, Biogen, Biohaven Pharmaceuticals, BioMarin Pharmaceutical Inc., BioXcel Therapeutics, Bloom Science Inc., BridgeBio Pharma Inc., Camp4 Therapeutics Corporation, Cerebral Therapeutics, Cerevel, Clinical Education Alliance, Coda Biotherapeutics, Corlieve Therapeutics, Eisai, Eliem Therapeutics, Encoded Therapeutics, Encoded Therapeutics, Engage Therapeutics, Engrail, Epalex, Epihunter, Epiminder, Epitel Inc., Equilibre BioPharmaceuticals, Greenwich Biosciences, Grin Therapeutics, GW Pharma, Janssen Phamaceutica, Jazz Pharmaceuticals, Knopp Biosciences, Lipocine, LivaNova, Longboard Pharmaceuticals, Lundbeck, Marinus, Mend Neuroscience, Marck, NeuCyte Inc., Neumirna Therapeutics, Neurocrine, Neuroelectives USA Corporation, Neuronetics Inc., Neuropace, NxGen Medicine Inc., Ono Pharmaceutical Co., Otsuka Pharmaceutical Development, Ovid Therapeutics Inc., Paladin Labs, Passage Bio, Pfizer, Praxis, Pure Tech LTY Inc., Rafa Laboratories Ltd, SK Life Sciences, Sofinnova, Stoke, Supernus, Synergia Medical, Takeda, UCB Inc., Ventus Therapeutics, Xenon, Xeris, Zogenix, Zynerba. Dr. French also has received research support from the Epilepsy Study Consortium (Funded by Andrews Foundation, Eisai, Engage, Lundbeck, Pfizer, SK Life Science, Sunovion, UCB, Vogelstein Foundation), the Epilepsy Study Consortium/Epilepsy Foundation (Funded by UCB), GW/FACES, and NINDS. She is on the editorial board of Lancet Neurology and Neurology Today. She is Chief Medical/Innovation Officer of the Epilepsy Foundation. She has received travel reimbursement related to research, advisory meetings, or presentation of results at scientific meetings from the Epilepsy Study Consortium, the Epilepsy Foundation, Angelini Pharma S.p.A., Clinical Education Alliance, NeuCyte, Inc., Neurocrine, Praxis, and Xenon. Stéphane Auvin is Deputy Editor for Epilepsia. He has served as consultant or gave lectures for Angelini, Biocodex, Eisai, Encoded, Grintherapeutics, Jazz Pharmaceuticals, Neuraxpharm, Orion, Nutricia, Proveca, UCB Pharma, Vitaflo, Xenon, Zogenix. He has been investigator for clinical trials for Eisai, Marinus, Proveca, Takeda, UCB Pharma and Zogenix. Dr. Van der Geyten is an employee of Janssen Research & Development, a division of Janssen Phamaceutica, Belgium, and holds stock in Johnson & Johnson companies. Dr. Kenney is employed full-time at Xenon Pharmaceuticals as Chief Medical Officer. Dr. Novak is a full-time employee of Janssen Research and Development. We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines. Dr. Fountain has received clinical trial grants to the University of Virginia from UCB, SK Lifesciences, Xenon, Neurelis, Medtronic, and InSightec and is an independent director and holds stock at Acumen Pharmaceuticals and Hexokine Therapeutics, and consults and receives stock options at Shackleton Pharma. Dr. Grzeskowiak was an employee of the Epilepsy Foundation. We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Figures
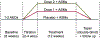
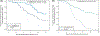
References
-
- Romero J and Goldenholz DM, Statistical efficiency of patient data in randomized clinical trials of epilepsy treatments adds value. Epilepsia, 2020. 61(10): p. 2323–2324. - PubMed
-
- French JA, et al., Designing a new proof-of-principle trial for treatment of partial seizures to demonstrate efficacy with minimal sample size and duration-a case study. Epilepsy Res, 2013. 106(1–2): p. 230–6. - PubMed
-
- Ryvlin P, Cucherat M, and Rheims S, Risk of sudden unexpected death in epilepsy in patients given adjunctive antiepileptic treatment for refractory seizures: a meta-analysis of placebo-controlled randomised trials. Lancet Neurol, 2011. 10(11): p. 961–8. - PubMed
-
- Mahmoud AA, et al., Ineffectiveness of topiramate and levetiracetam in infantile spasms non-responsive to steroids. Open labeled randomized prospective study. Neurosciences (Riyadh), 2013. 18(2): p. 143–6. - PubMed

