Initial Clinical Experience With AneuFix Injectable Biocompatible Elastomer for Translumbar Embolization of Type 2 Endoleaks
- PMID: 37073926
- PMCID: PMC11707960
- DOI: 10.1177/15266028231165731
Initial Clinical Experience With AneuFix Injectable Biocompatible Elastomer for Translumbar Embolization of Type 2 Endoleaks
Abstract
Purpose: The aim of this study was to assess the initial experience, technical success, and clinical benefit of AneuFix (TripleMed, Geleen, the Netherlands), a novel biocompatible and non-inflammatory elastomer that is directly injected into the aneurysm sac by a translumbar puncture in patients with a type II endoleak and a growing aneurysm.
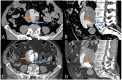
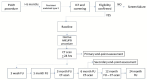
Materials and methods: A multicenter, prospective, pivotal study was conducted (ClinicalTrials.gov:NCT02487290). Patients with a type II endoleak and aneurysm growth (>5 mm) were included. Patients with a patent inferior mesenteric artery connected to the endoleak were excluded for initial safety reasons. The endoleak cavity was translumbar punctured with cone-beam computed tomography (CT) and software guidance. Angiography of the endoleak was performed, all lumbar arteries connected to the endoleak were visualized, and AneuFix elastomer was injected into the endoleak cavity and short segment of the lumbar arteries. The primary endpoint was technical success, defined as successful filling of the endoleak cavity with computed tomography angiography (CTA) assessment within 24 hours. Secondary endpoints were clinical success defined as the absence of abdominal aortic aneurysm (AAA) growth at 6 months on CTA, serious adverse events, re-interventions, and neurological abnormalities. Computed tomography angiography follow-up was performed at 1 day and at 3, 6, and 12 months. This analysis reports the initial experience of the first 10 patients treated with AneuFix.
Results: Seven men and 3 women with a median age of 78 years (interquartile range (IQR), 74-84) were treated. Median aneurysm growth after endovascular aneurysm repair (EVAR) was 19 mm (IQR, 8-23 mm). Technical success was 100%; it was possible to puncture the endoleak cavity of all treated patients and to inject AneuFix. Clinical success at 6 months was 90%. One patient showed 5 mm growth with persisting endoleak, probably due to insufficient endoleak filling. No serious adverse events related to the procedure or AneuFix material were reported. No neurological disorders were reported.
Conclusion: The first results of type II endoleak treatment with AneuFix injectable elastomer in a small number of patients with a growing aneurysm show that it is technically feasible, safe, and clinically effective at 6 months.
Clinical impact: Effective and durable embolization of type II endoleaks causing abdominal aortic aneurysms (AAA) growth after EVAR is challenging. A novel injectable elastic polymer (elastomer) was developed, specifically designed to treat type II endoleaks (AneuFix, TripleMed, Geleen, the Netherlands). Embolization of the type II endoleak was performed by translumbar puncture. The viscosity changes from paste-like during injection, into an elastic implant after curing. The initial experience of this multicentre prospective pivotal trial demonstrated that the procedure is feasible and safe with a technical success of 100%. Absence of AAA growth was observed in 9 out of 10 treated patients at 6 months.
Keywords: abdominal aortic aneurysm; abdominal aortic aneurysm, AAA growth; elastomer; embolization; endovascular aneurysm repair; polymer; translumbar; type II endoleak.
Conflict of interest statement
Declaration of Conflicting InterestsThe author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: M.J.J. is in the advisory board of TripleMed B.V. and patent holder of the elastomer formula of the AneuFix material.
Figures



Similar articles
-
Aortic aneurysm sac filling with AneuFix injectable polymer during endovascular aneurysm repair: feasibility and safety trial study protocol.BMJ Open. 2024 Jul 15;14(7):e082380. doi: 10.1136/bmjopen-2023-082380. BMJ Open. 2024. PMID: 39009453 Free PMC article.
-
Multicenter experience in translumbar type II endoleak treatment in the hybrid room with needle trajectory planning and fusion guidance.J Vasc Surg. 2020 Sep;72(3):1043-1049. doi: 10.1016/j.jvs.2019.10.076. Epub 2019 Dec 25. J Vasc Surg. 2020. PMID: 31882316
-
Surgical treatment patterns and clinical outcomes of patients treated for expanding aneurysm sacs with type II endoleaks after endovascular aneurysm repair.J Vasc Surg. 2021 Feb;73(2):484-493. doi: 10.1016/j.jvs.2020.05.062. Epub 2020 Jun 29. J Vasc Surg. 2021. PMID: 32615284
-
Preoperative Inferior Mesenteric Artery Embolization: A Valid Method to Reduce the Rate of Type II Endoleak after EVAR?Ann Vasc Surg. 2017 Feb;39:40-47. doi: 10.1016/j.avsg.2016.05.106. Epub 2016 Aug 12. Ann Vasc Surg. 2017. PMID: 27531083 Review.
-
Type II endoleak: a problem to be solved.J Cardiovasc Surg (Torino). 2014 Feb;55(1):109-18. J Cardiovasc Surg (Torino). 2014. PMID: 24356053 Review.
Cited by
-
Optimizing the Radiopacity of an Injectable Polymer on Fluoroscopy used for Treatment of Type II Endoleak After Endovascular Aneurysm Repair.Cardiovasc Eng Technol. 2025 Aug;16(4):377-385. doi: 10.1007/s13239-025-00779-w. Epub 2025 Mar 24. Cardiovasc Eng Technol. 2025. PMID: 40126785 Free PMC article.
-
Endovascular Repair of Spontaneous Rupture of Stent Graft Branch in Thoracoabdominal Aortic Aneurysm-Management, Case Study, and Review.J Clin Med. 2024 Dec 17;13(24):7687. doi: 10.3390/jcm13247687. J Clin Med. 2024. PMID: 39768610 Free PMC article.
-
Aortic aneurysm sac filling with AneuFix injectable polymer during endovascular aneurysm repair: feasibility and safety trial study protocol.BMJ Open. 2024 Jul 15;14(7):e082380. doi: 10.1136/bmjopen-2023-082380. BMJ Open. 2024. PMID: 39009453 Free PMC article.
-
Open Repair of a Large Abdominal Aortic Aneurysm With a Type 2 Endoleak After an Endovascular Aortic Aneurysm Repair: A Case Report and Literature Review.Cureus. 2023 Jun 12;15(6):e40315. doi: 10.7759/cureus.40315. eCollection 2023 Jun. Cureus. 2023. PMID: 37448430 Free PMC article.
References
-
- Avgerinos ED, Chaer RA, Makaroun MS. Type II endoleaks. J Vasc Surg. 2014;60(5):1386–1391. - PubMed
-
- Dijkstra ML, Zeebregts CJ, Verhagen HJM, et al.. Incidence, natural course, and outcome of type II endoleaks in infrarenal endovascular aneurysm repair based on the ENGAGE registry data. J Vasc Surg. 2020;71(3):780–789. - PubMed
-
- Veith FJ, Baum RA, Ohki T, et al.. Nature and significance of endoleaks and endotension: summary of opinions expressed at an international conference. J Vasc Surg. 2002;35(5):1029–1035. - PubMed
-
- Wanhainen A, Verzini F, Van Herzeele I, et al.. Editor’s choice—European Society for Vascular Surgery (ESVS) 2019 clinical practice guidelines on the management of abdominal aorto-iliac artery aneurysms. Eur J Vasc Endovasc Surg. 2019;57(1):8–93. - PubMed
-
- Wu WW, Swerdlow NJ, Dansey K, et al.. Surgical treatment patterns and clinical outcomes of patients treated for expanding aneurysm sacs with type II endoleaks after endovascular aneurysm repair. J Vasc Surg. 2021;73(2):484–493. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

