Persistent inflammation selectively activates opioid-sensitive phasic-firing neurons within the vlPAG
- PMID: 37073984
- PMCID: PMC10202481
- DOI: 10.1152/jn.00016.2023
Persistent inflammation selectively activates opioid-sensitive phasic-firing neurons within the vlPAG
Abstract
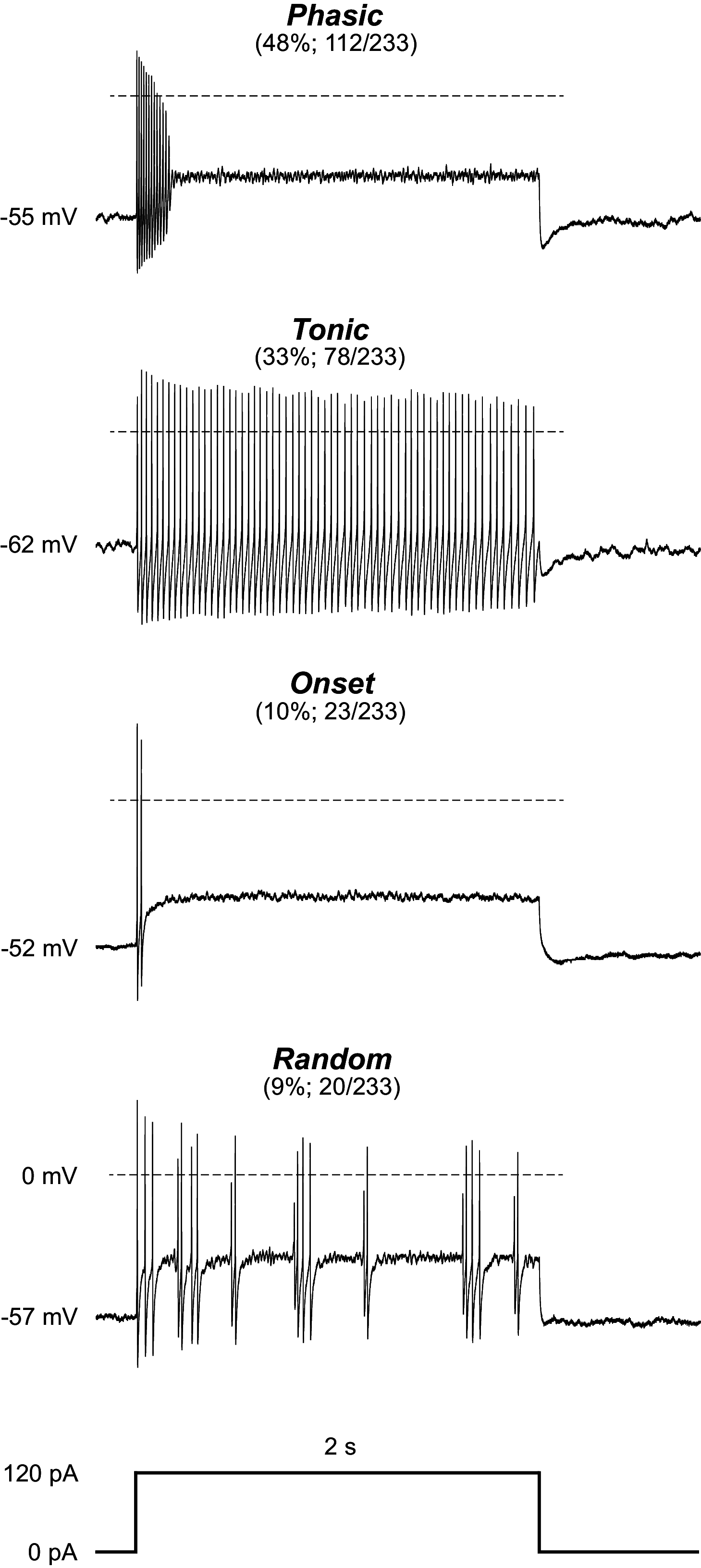
The ventrolateral periaqueductal gray (vlPAG) is a key brain area within the descending pain modulatory pathway and an important target for opioid-induced analgesia. The vlPAG contains heterogeneous neurons with respect to neurotransmitter content, receptor and channel expression, and in vivo response to noxious stimuli. This study characterizes intrinsic membrane properties of vlPAG neurons to identify neuron types that respond to inflammation and determine whether the pain-responsive neurons are inhibited by opioids. Surveying 382 neurons identified four neuron types with distinct intrinsic firing patterns: Phasic (48%), Tonic (33%), Onset (10%), and Random (9%). Mu-opioid receptor (MOR) expression was determined by the ability of a selective MOR agonist (DAMGO) to activate G protein-coupled inwardly rectifying potassium channel (GIRK) currents. Opioid-sensitive neurons were observed within each neuron type. Opioid sensitivity did not correlate with other intrinsic firing features, including low-threshold spiking that has been previously proposed to identify opioid-sensitive GABAergic neurons in the vlPAG of mice. Complete Freund's adjuvant (CFA)-induced acute inflammation (2 h) had no effect on vlPAG neuron firing patterns. However, persistent inflammation (5-7 days) selectively activated Phasic neurons through a significant reduction in their firing threshold. Opioid-sensitive neurons were strongly activated compared with the opioid-insensitive Phasic neurons. Overall, this study provides a framework to further identify neurons activated by persistent inflammation so that they may be targeted for future pain therapies.NEW & NOTEWORTHY Intrinsic firing properties define four distinct vlPAG neuron populations, and a subset of each population expresses MORs coupled to GIRK channels. Persistent, but not acute, inflammation selectively activates opioid-sensitive Phasic vlPAG neurons. Although the vlPAG is known to contribute to the descending inhibition of pain, the activation of a single physiologically defined neuron type in the presence of persistent inflammation represents a mechanism by which the vlPAG participates in descending facilitation of pain.
Keywords: descending pain modulation; electrophysiology; firing properties; inflammatory pain; vlPAG.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures





References
-
- Heinricher MM, Ingram SL. Brainstem and nociceptive modulation. In: The Senses: A Comprehensive Reference (2nd ed.), edited by Fritzsch B. Oxford, UK: Elsevier, 2020, vol. 5, p. 249–271.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

