Multi-technique structural analysis of zinc carboxylates (soaps)
- PMID: 37073995
- PMCID: PMC10167895
- DOI: 10.1039/d3dt00184a
Multi-technique structural analysis of zinc carboxylates (soaps)
Abstract
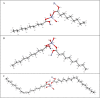
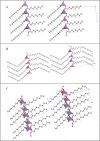
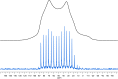
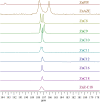
A series of medium- and long-chain zinc carboxylates (zinc octanoate, zinc nonanoate, zinc decanoate, zinc undecanoate, zinc dodecanoate, zinc pivalate, zinc stearate, zinc palmitate, zinc oleate, and zinc azelate) was analyzed by ultra-high-field 67Zn NMR spectroscopy up to 35.2 T, as well as 13C NMR and FTIR spectroscopy. We also report the single-crystal X-ray diffraction structures of zinc nonanoate, zinc decanoate, and zinc oleate-the first long-chain carboxylate single-crystals to be reported for zinc. The NMR and X-ray diffraction data suggest that the carboxylates exist in three distinct geometric groups, based on structural and spectroscopic parameters. The ssNMR results presented here present a future for dynamic nuclear polarization (DNP)-NMR-based minimally invasive methods for testing artwork for the presence of zinc carboxylates.
Conflict of interest statement
There are no conflicts of interest to declare.
Figures



References
Grants and funding
LinkOut - more resources
Full Text Sources

