Sulfur-Polymer Nanoparticles: Preparation and Antibacterial Activity
- PMID: 37074085
- PMCID: PMC10165599
- DOI: 10.1021/acsami.3c03826
Sulfur-Polymer Nanoparticles: Preparation and Antibacterial Activity
Abstract
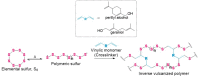
High sulfur content polymers prepared by inverse vulcanization have many reported potential applications, including as novel antimicrobial materials. High sulfur content polymers usually have limited water-solubility and dispersibility due to their hydrophobic nature, which could limit the development of their applications. Herein, we report the formulation of high sulfur content polymeric nanoparticles by a nanoprecipitation and emulsion-based method. High sulfur content polymeric nanoparticles were found to have an inhibitory effect against important bacterial pathogens, including Gram-positive methicillin-resistant Staphylococcus aureus and Gram-negative Pseudomonas aeruginosa. Salt-stable particles were formulated with the addition of a surfactant, which did not inhibit the antibacterial activity of the polymeric particles. Furthermore, the polymeric nanoparticles were found to inhibit S. aureus biofilm formation and exhibited low cytotoxicity against mammalian liver cells. Interaction of the polymeric particles with cellular thiols could be a potential mechanism of action against bacterial cells, as demonstrated by reaction with cysteine as a model thiol. The findings presented demonstrate methods of preparing aqueous dispersions of high sulfur content polymeric nanoparticles that could have useful biological applications.
Keywords: antibacterial; biofilm inhibition; inverse vulcanization; nanoparticles; polysulfides; sulfur.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
-
- Chung W. J.; Griebel J. J.; Kim E. T.; Yoon H.; Simmonds A. G.; Ji H. J.; Dirlam P. T.; Glass R. S.; Wie J. J.; Nguyen N. A.; Guralnick B. W.; Park J.; Somogyi Á.; Theato P.; Mackay M. E.; Sung Y.-E.; Char K.; Pyun J. The Use of Elemental Sulfur as an Alternative Feedstock for Polymeric Materials. Nat. Chem. 2013, 5 (6), 518–524. 10.1038/nchem.1624. - DOI - PubMed
-
- Gomez I.; Mecerreyes D.; Blazquez J. A.; Leonet O.; Ben Youcef H.; Li C.; Gómez-Cámer J. L.; Bondarchuk O.; Rodriguez-Martinez L. Inverse Vulcanization of Sulfur with Divinylbenzene: Stable and Easy Processable Cathode Material for Lithium-Sulfur Batteries. J. Power Sources 2016, 329, 72–78. 10.1016/j.jpowsour.2016.08.046. - DOI
-
- Smith J. A.; Green S. J.; Petcher S.; Parker D. J.; Zhang B.; Worthington M. J. H.; Wu X.; Kelly C. A.; Baker T.; Gibson C. T.; Campbell J. A.; Lewis D. A.; Jenkins M. J.; Willcock H.; Chalker J. M.; Hasell T. Crosslinker Copolymerization for Property Control in Inverse Vulcanization. Chem. Eur. J. 2019, 25 (44), 10433–10440. 10.1002/chem.201901619. - DOI - PubMed
-
- Hoefling A.; Lee Y. J.; Theato P. Sulfur-Based Polymer Composites from Vegetable Oils and Elemental Sulfur: A Sustainable Active Material for Li–S Batteries. Macromol. Chem. Phys. 2017, 218 (1), 1600303.10.1002/macp.201600303. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

