Maternal Bacterial Engraftment in Multiple Body Sites of Cesarean Section Born Neonates after Vaginal Seeding-a Randomized Controlled Trial
- PMID: 37074174
- PMCID: PMC10294643
- DOI: 10.1128/mbio.00491-23
Maternal Bacterial Engraftment in Multiple Body Sites of Cesarean Section Born Neonates after Vaginal Seeding-a Randomized Controlled Trial
Abstract
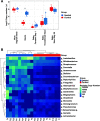
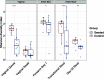
Children delivered by elective, prelabor Cesarean section (C-section) are not exposed to the birth canal microbiota and, in relation to vaginally delivered children, show altered microbiota development. Perturbed microbial colonization during critical early-life windows of development alters metabolic and immune programming and is associated with an increased risk of immune and metabolic diseases. In nonrandomized studies, vaginal seeding of C-section-born neonates partially restores their microbiota colonization to that of their vaginally delivered counterparts, but without randomization, confounding factors cannot be excluded. In a double-blind, randomized, placebo-controlled trial, we determined the effect of vaginal seeding versus placebo seeding (control arm) on the skin and stool microbiota of elective, prelabor C-section-born neonates (n = 20) at 1 day and 1 month after birth. We also examined whether there were between-arm differences in engraftment of maternal microbes in the neonatal microbiota. In relation to the control arm, vaginal seeding increased mother-to-neonate microbiota transmission and caused compositional changes and a reduction in alpha diversity (Shannon Index) of the skin and stool microbiota. The neonatal skin and stool microbiota alpha diversity when maternal vaginal microbiota is provided is intriguing and highlights the need of larger randomized studies to determine the ecological mechanisms and effects of vaginal seeding on clinical outcomes. IMPORTANCE Children delivered by elective C-section are not exposed to the birth canal and show altered microbiota development. Impairing microbial colonization during early life alters metabolic and immune programming and is associated with an increased risk of immune and metabolic diseases. In a double-blind, randomized, placebo-controlled trial, we determined the effect of vaginal seeding on the skin and stool microbiota of elective C-section born neonates and found that vaginal seeding increased mother-to-neonate microbiota transmission and caused compositional changes and a reduction in the skin and stool microbiota diversity. The reduction of neonatal skin and stool microbiota diversity when maternal vaginal microbiota is provided is intriguing and highlights the need of larger randomized studies to determine the ecological mechanisms and effects of vaginal seeding on clinical outcomes.
Keywords: Cesarean section; microbiome; microbiota; neonate; randomized controlled trial; vaginal microbiome transfer; vaginal seeding.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
The influence of maternal gut and vaginal microbiota on gastrointestinal colonization of neonates born vaginally and per caesarean section.BMC Pregnancy Childbirth. 2025 Mar 8;25(1):254. doi: 10.1186/s12884-025-07358-w. BMC Pregnancy Childbirth. 2025. PMID: 40057706 Free PMC article.
-
Oral administration of maternal vaginal microbes at birth to restore gut microbiome development in infants born by caesarean section: A pilot randomised placebo-controlled trial.EBioMedicine. 2021 Jul;69:103443. doi: 10.1016/j.ebiom.2021.103443. Epub 2021 Jun 27. EBioMedicine. 2021. PMID: 34186487 Free PMC article. Clinical Trial.
-
Maternal bacteria to correct abnormal gut microbiota in babies born by C-section.Medicine (Baltimore). 2020 Jul 24;99(30):e21315. doi: 10.1097/MD.0000000000021315. Medicine (Baltimore). 2020. PMID: 32791721 Free PMC article. Clinical Trial.
-
Crucial nuances in understanding (mis)associations between the neonatal microbiome and Cesarean delivery.Trends Mol Med. 2022 Oct;28(10):806-822. doi: 10.1016/j.molmed.2022.07.005. Epub 2022 Sep 6. Trends Mol Med. 2022. PMID: 36085277 Free PMC article. Review.
-
Unveiling the neonatal gut microbiota: exploring the influence of delivery mode on early microbial colonization and intervention strategies.Arch Gynecol Obstet. 2024 Dec;310(6):2853-2861. doi: 10.1007/s00404-024-07843-1. Epub 2024 Nov 26. Arch Gynecol Obstet. 2024. PMID: 39589476 Review.
Cited by
-
The skin microbiome in pediatric atopic dermatitis and food allergy.Allergy. 2024 Jun;79(6):1470-1484. doi: 10.1111/all.16044. Epub 2024 Feb 3. Allergy. 2024. PMID: 38308490 Free PMC article. Review.
-
Vaginal delivery provides skin colonization resistance from environmental microbes in the NICU.Clin Transl Med. 2023 Dec;13(12):e1506. doi: 10.1002/ctm2.1506. Clin Transl Med. 2023. PMID: 38058267 Free PMC article. No abstract available.
-
Clinical translation of microbiome research.Nat Med. 2025 Apr;31(4):1099-1113. doi: 10.1038/s41591-025-03615-9. Epub 2025 Apr 11. Nat Med. 2025. PMID: 40217076 Review.
-
Impact of Maternal Microbiota Composition on Neonatal Immunity and Early Childhood Allergies: A Systematic Review.Pediatr Rep. 2025 Jun 17;17(3):67. doi: 10.3390/pediatric17030067. Pediatr Rep. 2025. PMID: 40559462 Free PMC article. Review.
-
Inspecting mother-to-infant microbiota transmission: disturbance of strain inheritance by cesarian section.Front Microbiol. 2024 Feb 29;15:1292377. doi: 10.3389/fmicb.2024.1292377. eCollection 2024. Front Microbiol. 2024. PMID: 38486699 Free PMC article.
References
-
- Cox LM, Yamanishi S, Sohn J, Alekseyenko AV, Leung JM, Cho I, Kim SG, Li H, Gao Z, Mahana D, Zárate Rodriguez JG, Rogers AB, Robine N, Loke P, Blaser MJ. 2014. Altering the intestinal microbiota during a critical developmental window has lasting metabolic consequences. Cell 158:705–721. doi:10.1016/j.cell.2014.05.052. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

